Figure 7.
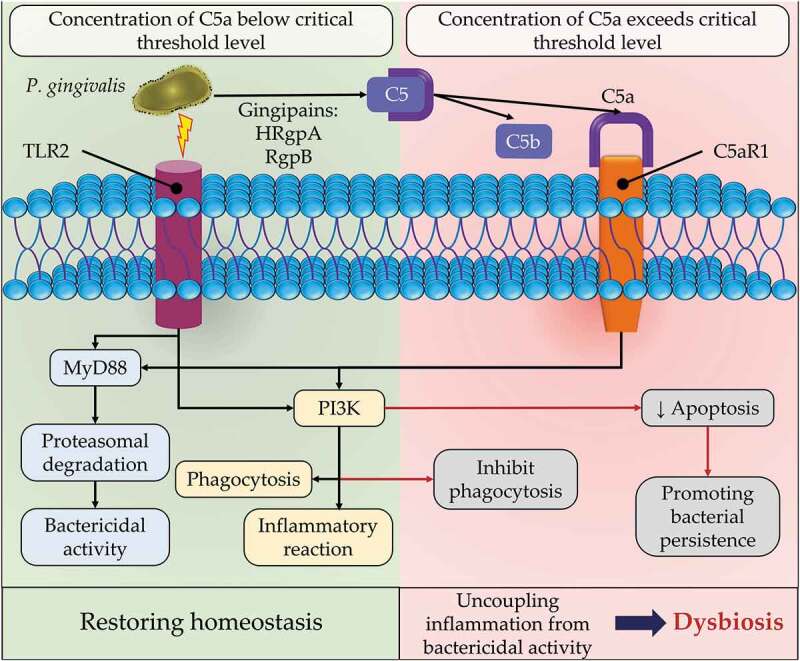
Porphyromonas gingivalis enhancing dysbiosis through uncoupling of inflammation from bactericidal activity of the phagocytic cells. P. gingivalis interacts with Toll-like receptor (TLR2), and acts on complement component 5 (C5) through P. gingivalis-associated arginine gingipains (HRgpA and RgpB) to produce C5a and C5b. C5a ligand then interacts with its specific complement C5a receptor (C5ar1) that together are co-activated with TLR2 on the surface of phagocytic cells. The cross-reactivity of both receptors could induce myeloid differentiation primary response 88 (MYD88)-induced inflammation or be blocked if MyD88 is inactivated. However, the same cross-reactivity of TLR2-C5aR1 complex could bypass MyD88 and induce the phosphoinositide 3-kinases (PI3K) pathway that may induce inflammation in phagocytic cells. In a similar manner, the activated PI3K could inhibit bacterial phagocytosis/apoptosis and supress phagolysosomal maturation, enhancing bacterial persistence. The latter mechanism is dependent on increased concentration of C5a beyond a threshold level (100 nM). The insurance of bacterial survival while inducing inflammation results in increased inflammophilic pathobionts and enhances dysbiosis.
