Low-grade gliomas (LGG) represent the most common type of central nervous system (CNS) neoplasm in children.1–3 Importantly, pediatric-type LGGs, affecting children and young adults, are molecularly distinct from adult-type LGGs. While the latter typically harbor IDH1 or IDH2 mutations, pediatric-type LGGs invariably involve activation of the mitogen‑activated protein kinase (MAPK) cascade.4,5 While adult-type LGG often progress into higher grade gliomas over time, pediatric-type LGGs are associated with excellent overall survival of >95%.4 Nonetheless, they are at risk for progression many years after diagnosis and patients may suffer functional morbidity.
The most common alteration leading to MAPK activation is the KIAA1549::BRAF fusion involving exon 16 of KIAA1549 and exon 9 of BRAF, and 14% of pediatric type low-grade glioma occur in individuals with neurofibromatosis type 1.4,6,7 Many other molecular alterations have been described.4 Here, we present a case of pediatric-type LGG characterized by an unreported fusion event between MYO5A and FGFR1.
Case
A previously healthy 15-year-old female without pertinent family history presented to medical attention with headache and a one month history of progressive right sided weakness and sensory deficit. Imaging revealed a left frontoparietal mass with an unremarkable spine MRI (Figure 1). She underwent gross total resection of the lesion with a combination of microsurgical technique and ultrasonic aspirator. Care was taken to protect the neighboring eloquent area dorsally, and we were able to identify a resection plane circumferentially. Of note, the abnormal appearing tissue had also occasional areas of hematoma which suggested recent hemorrhage. Histologic sections revealed a biphasic glioma with predominant myxoid histology in addition to regions with a more solid piloid appearance (Figure 2). The tumor cells were positive for GFAP and Olig2, negative for IDH1-R132H, EMA, and CD34, and showed retained expression of ATRX. The Ki-67 index was low overall, with focally increased labeling of up to 15% of tumor cell nuclei, corresponding with up to 2 mitoses per 10 high-power fields. Evaluation by an anchored multiplex PCR next-generation sequencing fusion panel identified a novel MYO5A::FGFR1 fusion, joining exon 25 of MYO5A with exon 10 of FGFR1. This in-frame fusion includes the entire C-terminal protein kinase domain of FGFR1 similar to other types of driver FGFR rearrangements described previously in glioma.14 Whole genome methylation profiling, matched the tumor to methylation class supratentorial pilocytic astrocytoma using both the NCI EPIC methylation classifier and version 12.6 of the Heidelberg classifier, leading to a final integrated diagnosis of pilocytic astrocytoma (CNS WHO grade 1). Finally, confirmation of MAPK activation was achieved via immunohistochemistry for phosphorylated ERK (pERK), demonstrating similar degree of staining to a positive control low-grade glioma sample characterized by a KIAA1549::BRAF fusion (Figure 2D).
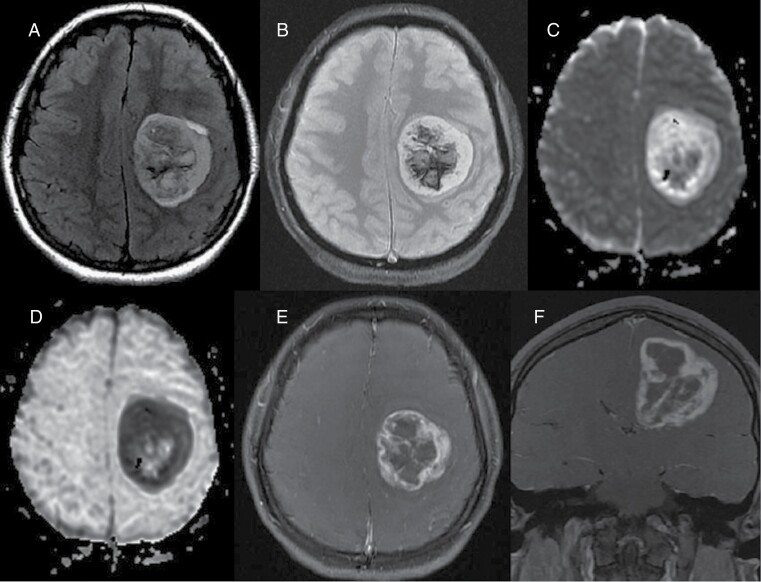
Figure 1.
Preoperative images. (A) Axial FLAIR image shows 5 cm mass centered in the left centrum semiovale and corona radiata with surrounding minimal vasogenic edema. (B) On T2 GRE, there is central susceptibility compatible with internal hemorrhage. (C) On diffusion weighted image, the peripheral components show mostly isointense signal with increased signal in the lateral margin. Internal heterogeneity, likely due to internal blood products. (D) ADC map shows corresponding bright signal in the peripheral regions with dark signal in the lateral margin, suggestive of facilitated diffusion and low cellularity mostly with relative diffusion restriction in the lateral margin. Heterogeneous signal at the center was due to blood products. Fat-saturated contrast enhanced axial (E) and coronal (F) images show avid thick enhancement in the peripheral solid component with irregular lobulated contours and bell-pepper like appearance. Overall, MRI features are suggestive of an aggressive high-grade glial tumor except the diffusion weighted images, demonstrating facilitated diffusion in the most of the solid peripheral components with small focal diffusion restriction in the lateral margin.
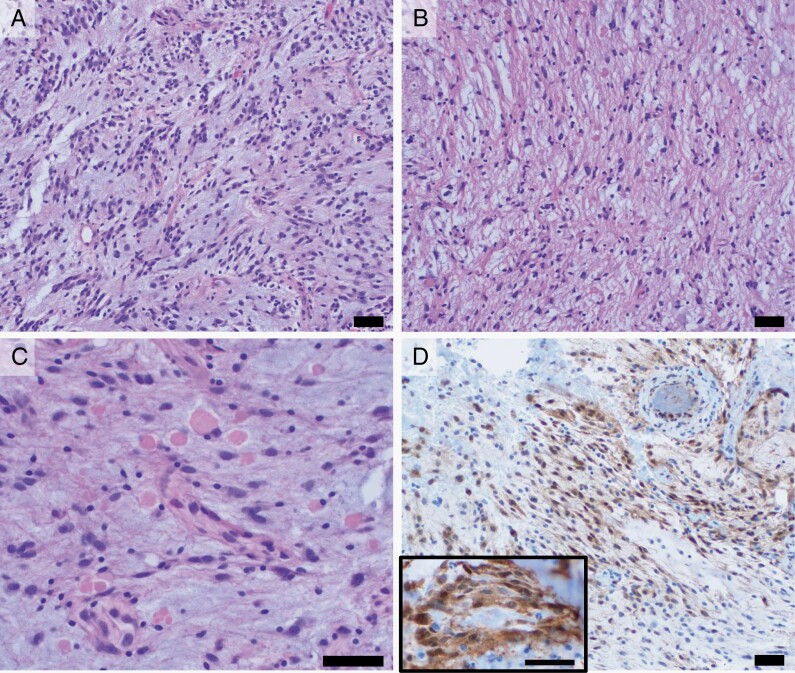
Figure 2.
(H&E, 200×) Histologic sections show a predominant component with often elongated tumor cells with occasional bipolar cytoplasmic processes in a myxoid background (A), in addition to a more solid, fibrillary component (B). C. (H&E, 400×) Both components showed frequent eosinophilic granular bodies. D. (pERK, 200×) Increased cytoplasmic and nuclear immunohistochemical staining for pERK is seen in tumor cells, indicative of MAPK pathway activation. Staining is comparable to that seen in a separate example of pilocytic astrocytoma with KIAA1549::BRAF fusion provided for reference (inset, 400×). Scale bars = 50 µm.
With post-operative physical therapy, the patient has had near resolution of her baseline neurologic deficits. She has been managed with observation and remains free of progression at her 4-month post-operative disease evaluation (Figure 3).
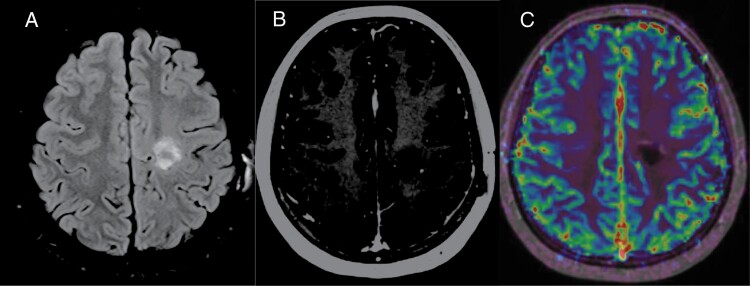
Figure 3.
Postoperative 4-month follow-up MRI. (A) Axial FLAIR shows hyperintense proteinaceous fluid containing resection cavity without any surrounding vasogenic edema. Mild expected gliosis surrounding the resection cavity. (B) Contrast enhanced axial T1-MPRAGE shows no significant worrisome enhancement. Minimal marginal enhancement expected in the postoperative stage. (C) Axial fused DSC perfusion image in a different slice shows lack of increased perfusion even in marginal thin enhancing areas, suggestive of gross total resection with post-operative changes.
Discussion
This case of a previously unreported FGFR1 fusion event highlights the increasingly important role that next-generation sequencing has in the diagnostic evaluation of pediatric brain tumors. This specific fusion has not been reported in the FusionGDB or the TCGA Fusion Gene Database but is nonetheless associated with glioma pathogenesis in this case as the entire protein kinase domain of FGFR1 was present in the unique rearrangement described. FGFR1 alterations, which most commonly include activating mutations, fusion events with TACC1, and tyrosine-kinase domain duplications, lead to MAPK activation upstream of RAF. For this reason, they can also lead to activation of the PI3K/mTOR pathway.8
The shared biology of MAPK activation in pediatric-type LGGs has led to the success of targeted therapy in the clinical setting. Selumetinib, a MEK1/2 inhibitor, is active in pediatric-type LGG9 and is currently being investigated in newly diagnosed pediatric LGG in the phase III setting comparing the safety and efficacy of selumetinib against standard of care carboplatin/vincristine chemotherapy (NCT04166409), which our patient would be eligible for in the setting of progression. For patients with recurrence who previously received tumor-directed treatment, selumetinib is also being studied as monotherapy against the combination of selumetinib and vinblastine (NCT04576117). Of note, MYO5A has previously been reported as a fusion partner for NTRK3 in a CNS ganglioneuroblastoma.12 The only other reported incidence of MYO5A::FGFR1 has occurred in the context of Spitz neoplasms, a subset of skin neoplasms.13 This suggests that a subset of these neoplasms may additionally benefit from MAPK targeted inhibition. Given the role of targeted therapy in the management of these gliomas, identifying unique MAPK-activating alterations will change management for adolescent and young adult patients harboring IDH-wild-type low-grade gliomas.10,11 This case resulted in the identification of a novel fusion event not previously reported in the context of low-grade glioma.
Contributor Information
Robert T Galvin, Division of Pediatric Hematology and Oncology and Bone Marrow Transplant, University of Minnesota, Minneapolis, MN, USA.
Cynthia Zheng, University of Minnesota Medical School, Minneapolis, MN, USA.
Garrett Fitzpatrick, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA.
Colleen L Forster, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA.
Carolina Sandoval-Garcia, Department of Neurosurgery, University of Minnesota, Minneapolis, MN, USA.
Daniel Guillaume, Department of Neurosurgery, University of Minnesota, Minneapolis, MN, USA.
Ahmed Elbermawy, Department of Neurosurgery, University of Minnesota, Minneapolis, MN, USA.
Andrew C Nelson, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA.
Can Özütemiz, Department of Radiology, University of Minnesota Medical School, Minneapolis, MN, USA.
Liam Chen, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA.
Christopher L Moertel, Division of Pediatric Hematology and Oncology and Bone Marrow Transplant, University of Minnesota, Minneapolis, MN, USA.
Conflict of Interest Statement
All authors have no conflicts of interest to disclose.
References
- 1. Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol. Biomarkers Prev. 2014;23(12):2716–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adel Fahmideh M, Scheurer ME.. Pediatric brain tumors: descriptive epidemiology, risk factors, and future directions. Cancer Epidemiol. Biomarkers Prev. 2021;30(5):813–821. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryall S, Zapotocky M, Fukuoka K, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas (Adults). N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Faulkner C, Ellis HP, Shaw A, et al. BRAF fusion analysis in pilocytic astrocytomas: KIAA1549-BRAF 15-9 fusions are more frequent in the midline than within the cerebellum. J Neuropathol Exp Neurol. 2015;74(9):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galvin R, Watson AL, Largaespada DA, et al. Neurofibromatosis in the era of precision medicine: development of MEK inhibitors and recent successes with selumetinib. Curr Oncol Rep. 2021;23(4):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryall S, Tabori U, Hawkins C.. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun. 2020;8(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lim-Fat MJ, Macdonald M, Lapointe S, et al. Molecular testing for adolescent and young adult central nervous system tumors: a Canadian guideline. Front Oncol. 2022;12:960509. https://www.frontiersin.org/articles/10.3389/fonc.2022.960509/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouffet E, Geoerger B, Moertel C, et al. Efficacy and safety of trametinib monotherapy or in combination with dabrafenib in pediatric BRAF V600–mutant Low-grade glioma. JCO. 2023;41(3):664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ito J, Nakano Y, Shima H, et al. Central nervous system ganglioneuroblastoma harboring MYO5A-NTRK3 fusion. Brain Tumor Pathol. 2020;37:105–110. https://link.springer.com/article/10.1007/s10014-020-00371-1 [DOI] [PubMed] [Google Scholar]
- 13. Quan VL, Zhang B, Zhang Y, et al. Integrating next-generation sequencing with morphology improves prognostic and biologic classification of spitz neoplasms. J Investig Dermatol. 2020;140(8):1599–1608. [DOI] [PubMed] [Google Scholar]
- 14. Roosen M, Odé Z, Bunt J, Kool M.. The oncogenic fusion landscape in pediatric CNS neoplasms. Acta Neuropathol. 2022 Apr;143(4):427–451. [DOI] [PMC free article] [PubMed] [Google Scholar]