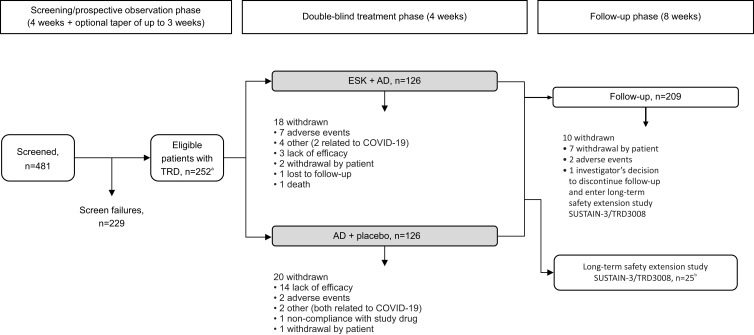
Figure 1.
Patient flow diagram.
Notes: aOnly patients with non-response to at least 2 AD treatments prior to randomization were eligible to participate in the study. bTwenty-four patients entered SUSTAIN-3/TRD3008 from the double-blind treatment phase of TRD3006; 1 patient entered SUSTAIN-3/TRD3008 from the follow-up phase of TRD3006.
Abbreviations: AD, antidepressant; ESK, esketamine; TRD, treatment-resistant depression.