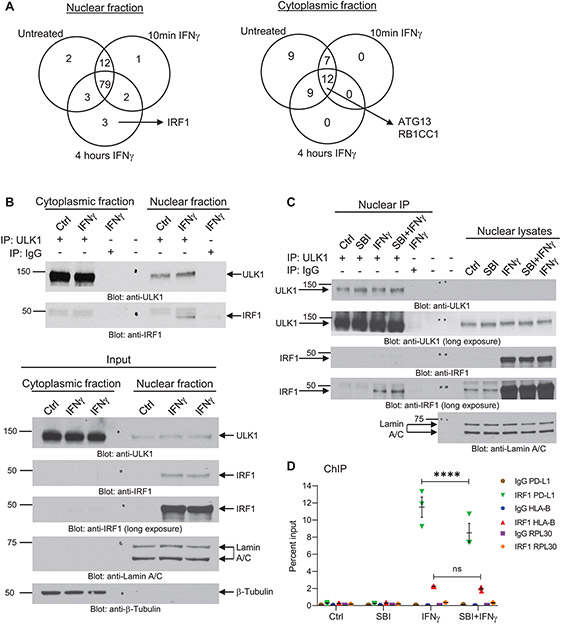
Figure 4. ULK1 binds IRF1 in the nucleus and controls IFNγ-induced IRF1 binding to the promoter region of PD-L1.
(A) A375 cells were either left untreated or were treated with IFNγ for 10 min (5000 IU/mL) or 4 hours (2500 IU/mL), followed by cytoplasmic and nuclear cell fractionation (Supplementary Fig. S5A). ULK1-protein complexes were co-immunoprecipitated using an anti-ULK1 monoclonal specific antibody conjugated to sepharose beads for each compartment/treatment condition. As negative control, the same procedure was followed, but using a rabbit monoclonal antibody IgG isotype control conjugated to sepharose beads instead of the anti-ULK1 antibody for the 4 hours (240 min) IFNγ treatment condition (see also Supplementary Fig. S5B). Protein complexes were eluted from the beads and submitted for nano-liquid chromatography-tandem mass spectrometry analysis. Venn diagrams show the number of putative ULK1 interacting proteins in the nuclear and cytoplasmic fractions under the three different treatment conditions. Proteins found on the negative control group were removed from the analysis. (B) A375 cells were either left untreated (Ctrl) or were treated with IFNγ for 4 hours (2500 IU/mL), followed by cytoplasmic and nuclear cell fractionation, as indicated. (Top panel) ULK1-protein complexes were co-immunoprecipitated using an anti-ULK1 monoclonal specific antibody and protein G sepharose beads for each compartment/treatment condition (IP: ULK1). As negative control, the same procedure was followed, but using a rabbit monoclonal antibody IgG isotype control instead of the anti-ULK1 antibody for the 4 hours IFNγ treatment condition (IP: IgG). Protein complexes were eluted from the beads and resolved by SDS-PAGE and immunoblotting analysis was performed for ULK1 and IRF1. (Bottom panel) Equal amounts of nuclear and cytoplasmic protein lysates for each treatment condition (Input) were resolved by SDS-PAGE and immunoblotting analysis was performed for ULK1 and IRF1. Lamin A/C and β-tubulin were used as loading control for the nuclear and cytoplasmic compartments, respectively. (C) A375 cells were pre-treated for 1 hour with either DMSO (Ctrl and IFNγ groups) or SBI-0206965 (SBI) (10 μM) followed by 6 hours of treatment with either DMSO (Ctrl), SBI (10 μM), IFNγ (2500 IU/mL) or SBI + IFNγ, as indicated. After treatment, cells were separated into nuclear and cytoplasmic fractions and ULK1-protein complexes were co-immunoprecipitated using an anti-ULK1 monoclonal specific antibody and protein G sepharose beads for each nuclear fraction treatment condition (IP: ULK1). As negative control, the same procedure was followed, but using a rabbit monoclonal antibody IgG isotype control instead of the anti-ULK1 antibody for the IFNγ treatment condition (IP: IgG). Protein complexes were eluted from the beads and resolved by SDS-PAGE and immunoblotting analysis was performed for ULK1 and IRF1. Equal amounts of nuclear protein lysates for each treatment condition were resolved on the same gel and immunoblotted for ULK1, IRF1 and Lamin A/C (loading control). (D) A375 cells were pre-treated for 1 hour with either DMSO (Ctrl and IFNγ groups) or SBI-0206965 (SBI) (10 μM) followed by 6 hours of treatment with either DMSO (Ctrl), SBI (10 μM), IFNγ (2500 IU/mL) or SBI + IFNγ, as indicated. ChIP assay was performed in A375 cells at the PD-L1 promoter, the HLA-B promoter, and the RPL30 promoter (negative control) for IRF1 binding. IgG antibody was used for each promoter region as negative control. Scatter dot plot shows data as percent enrichment relative to input ± SEM for three independent experiments. Statistical analyses were performed using two-way ANOVA followed by Tukey’s multiple comparisons test between treatment conditions for each antibody binding to each promoter region (IgG to PD-L1, HLA-B or RPL30 and IRF1 to PD-L1, HLA-B or RPL30). ****, p < 0.0001; ns, p > 0.05 between IFNγ and SBI + IFNγ treatment conditions.