Figure 1. Extracellular vesicles participation in the communication between glioblastoma cells and non-cancer cells in the neurogenic, immune, and perivascular tumor microenvironment.
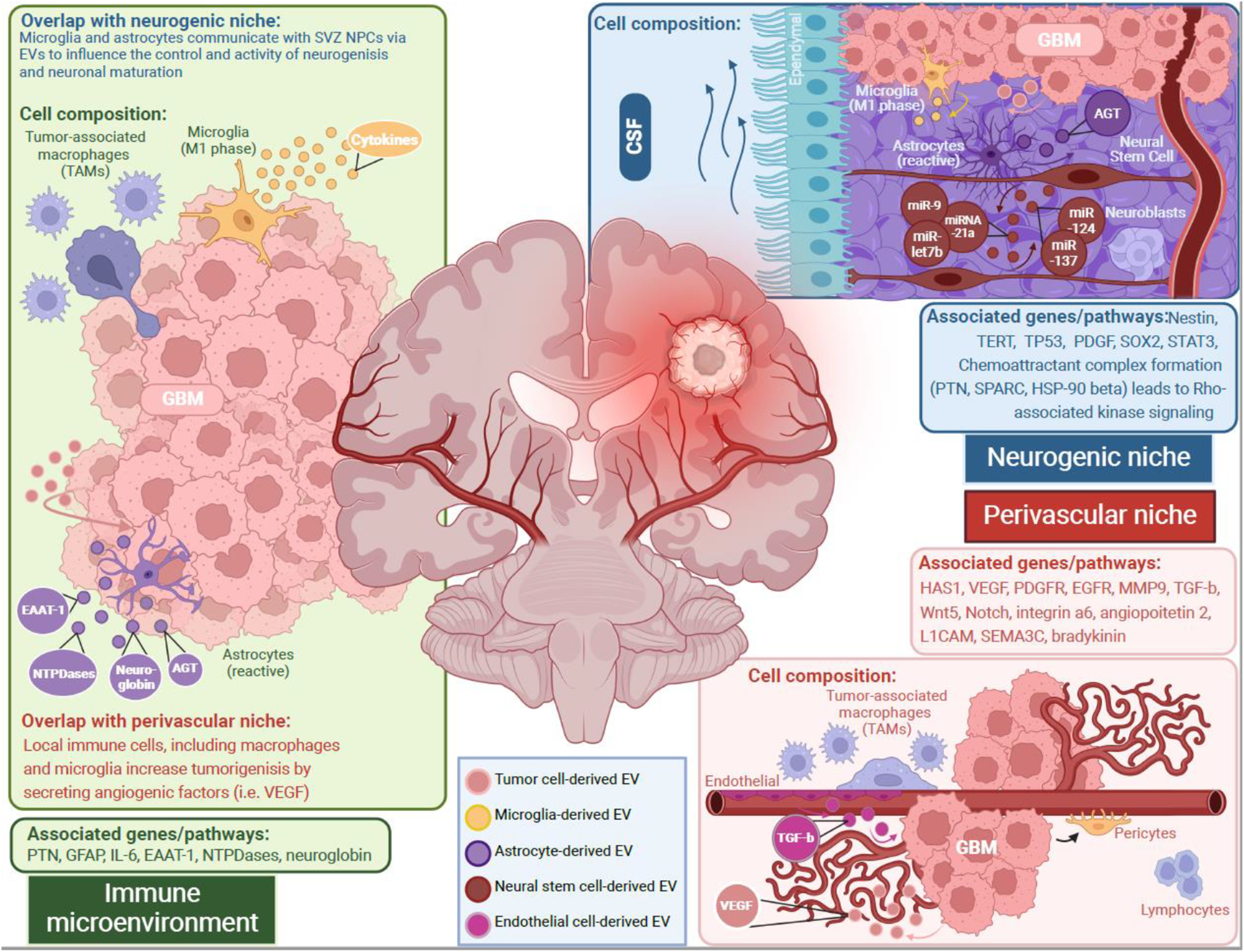
Each microenvironment presents niche-specific cell types and pathways that regulate tumor cell behavior. In each tumor microenvironment, there is reciprocal intercellular transport of proteins and microRNAs. Immune cells can release extracellular vesicles with cargo that impacts neural stem cell proliferation, such as cytokines from microglia extracellular vesicles which increase neural stem cell proliferation or EAAT-1 from activated astrocyte extracellular vesicles that decrease neural stem cell proliferation. These astrocytes can also increase tumor cell proliferation and survival with extracellular vesicles containing neuroglobin and alkylguanine DNA alkyltransferase respectively. Neural stem cells of the neurogenic niche can release extracellular that increase activated microglia and their cytokine release. Said neural stem cells also establish a crosstalk between themselves moderating quiescence and adult neurogenesis (miR-9, miRNA-21a, miR-let7b, miR-124, miR-137). Glioblastoma stem cells can manipulate this regulation by releasing extracellular vesicles that transform neural stem cells towards being cancerous. In the perivascular niche, endothelial cells have been seen to release extracellular vesicles containing TGF-β which prompts local glioblastoma stem cells towards differentiating into pericytes. Glioblastoma stem cells are also seen to recruit tumor-associated macrophages to increase tumor growth as well as supporting angiogenesis by increased VEGF.
