Figure 1.
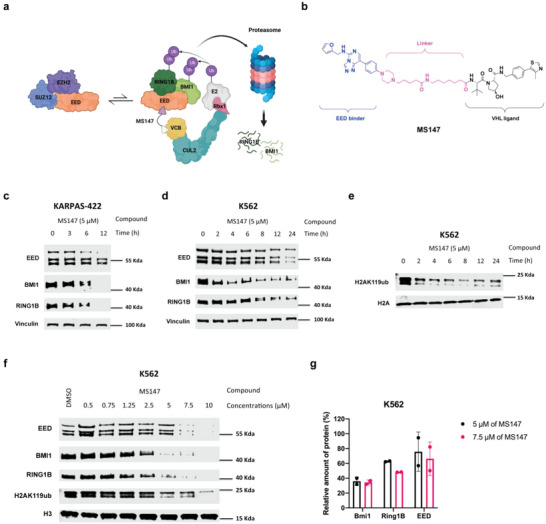
Discovery of the BMI1 and RING1B PROTAC MS147, which preferentially degrades BMI1 and RING1B over EED. a) The schematic of an EED‐binding PROTAC that preferentially degrades PRC1 components, BMI1 and RING1B, over EED, by hijacking the VHL‐Elongin C‐Elongin B (VCB) cullin‐2 (CUL2) RING E3 ligase complex. b) Chemical structure of MS147. c,d) Time‐dependent degradation of BMI1, RING1B, and EED induced by MS147 in KARPAS‐422 (c) and K562 (d) cells. KARPAS‐422 and K562 cells were treated with MS147 at 5 µm for the indicated time. The protein levels of EED, BMI1, and RING1B were determined by Western blotting (WB) with vinculin as the loading control. e) Time course of the H2AK119ub reduction induced by MS147 in K562 cells. K562 cells were treated with MS147 at 5 µm for the indicated time. The H2AK119ub protein level was determined by WB with H2A as the loading control. f) Concentration‐dependent degradation of BMI1, RING1B, and EED and reduction of H2AK119ub induced by MS147 in K562 cells. K562 cells were treated with MS147 at the indicated concentrations for 24 h. The protein levels of EED, BMI1, RING1B, and H2AK119ub were determined by WB with vinculin and H3 as the loading controls. g) Quantification of the EED, BMI1, and RING1B protein levels in K562 cells treated with 5 or 7.5 µm of MS147 for 24 h. The WB results shown in panels c–f are representative of two independent experiments.
