Figure 3.
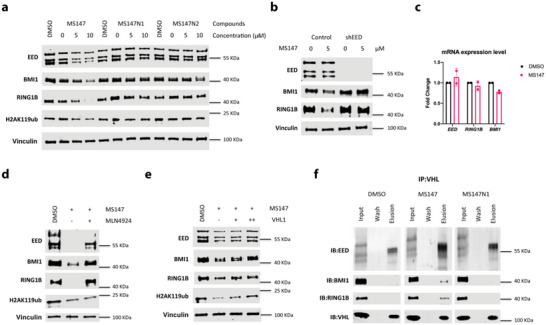
MS147 degrades BMI1 and RING1B in an EED‐, VHL‐, and ubiquitination‐dependent manner without altering mRNA expression levels. a) The effect of MS147, MS147N1, and MS147N2 on reducing the protein levels of EED, BMI1, RING1B, and H2AK119ub. K562 cells were treated with DMSO or the indicated compound at the indicated concentrations for 24 h. b) The effect of EED knockdown (KD) using shEED on rescuing MS147‐induced degradation of BMI1 and RING1B. K562 cells were transfected by lentivirus containing shEED or an empty vector for 24 h. The transfected cells were then treated with DMSO or MS147 (5 µ m) for 24 h. c) The effect of MS147 on the mRNA levels of EED, BMI1, and RING1B, determined by RT‐qPCR. KARPAS‐422 cells were treated with DMSO or MS147 (5 µ m) for 24 h. The mRNA levels were normalized to DMSO control. The data shown represent the means ± SD from two independent experiments. d,e) The effect of pretreatment with the neddylation inhibitor MLN4924 (d) or the VHL ligand VHL1 (e) on rescuing the degradation of BMI1 and RING1B induced by MS147. K562 cells were pretreated with MLN4924 (0.5 µ m) or VHL1 (+: 0.5 µ m; ++: 1 µ m) for 1 h, followed by treatment with MS147 (5 µ m) for 24 h. f) Coelution of EED‐BMI1‐RING1B with VHL by in vitro pulldown using a VHL antibody in the presence of MS147 or MS147N1. K562 cell lysates were treated with DMSO, MS147 (40 µ m) or MS147N1 (40 µ m) for 3 h. The protein levels of EED, BMI1, RING1B, and/or H2AK119ub in panels a, b, and d–f were determined by WB with vinculin as the loading control for (a), (b), (d), and (e). The WB results shown in panels a, b, and d–f are representative of two independent experiments.
