Figure EV5. Mechanistic model of TRPV2 control over metastatic melanoma cells dissemination through the dynamic regulation of nascent adhesion sites.
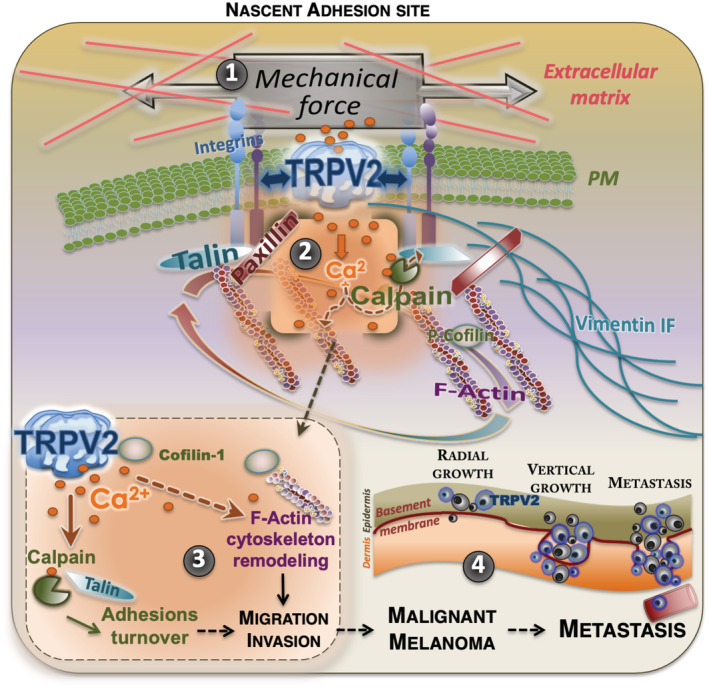
The essential role of TRPV2 in melanoma migration and invasion could be explained by the newly identified pro‐invasive properties of this mechanosensitive channel. Indeed, cancer cells and their associated microenvironment generate considerable mechanical forces applied onto the plasma membrane (PM) (1). These changes in PM tension regulate cell shape and movement. In malignant melanoma cells, the TRPV2 channel is recruited to the PM within paxillin‐containing early adhesion structures, and its constitutive activation elicits a subplasmalemmal localized Ca2+ ions uptake (2). TRPV2‐mediated Ca2+ influx triggers the activation of the intracellular Ca2+‐dependent cysteine protease, calpain (3). The cleavage of its substrate, the early adhesion protein talin linking membrane integrins and cytoskeleton, in fine prompts the disassembly of a subset of adhesion complexes and facilitates cell‐extracellular matrix (ECM) contact sites plasticity. Induction, selection, and maturation of nascent adhesion complexes at the cell leading edge serve as sampling the local ECM to select traction points producing forces that will drive the cell body forward. To further regulate the maturation of these adhesion structures along with the remodeling of the cytoskeleton, TRPV2 directly interacts with both the intermediate filament (IF) vimentin network, and the actin severing factor cofilin‐1, a central regulator of F‐actin dynamics. TRPV2‐induced signaling promotes the spatial and temporal accumulation of F‐actin bundles to improve advanced melanoma cell motility. Therefore, TRPV2 channel‐mediated Ca2+ influx tunes the plasticity of the melanoma tumor cell by locally controlling adhesion complexes maturation and cytoskeleton remodeling, potentiating the migratory and invasive behaviors of these malignant cells (4).
