-
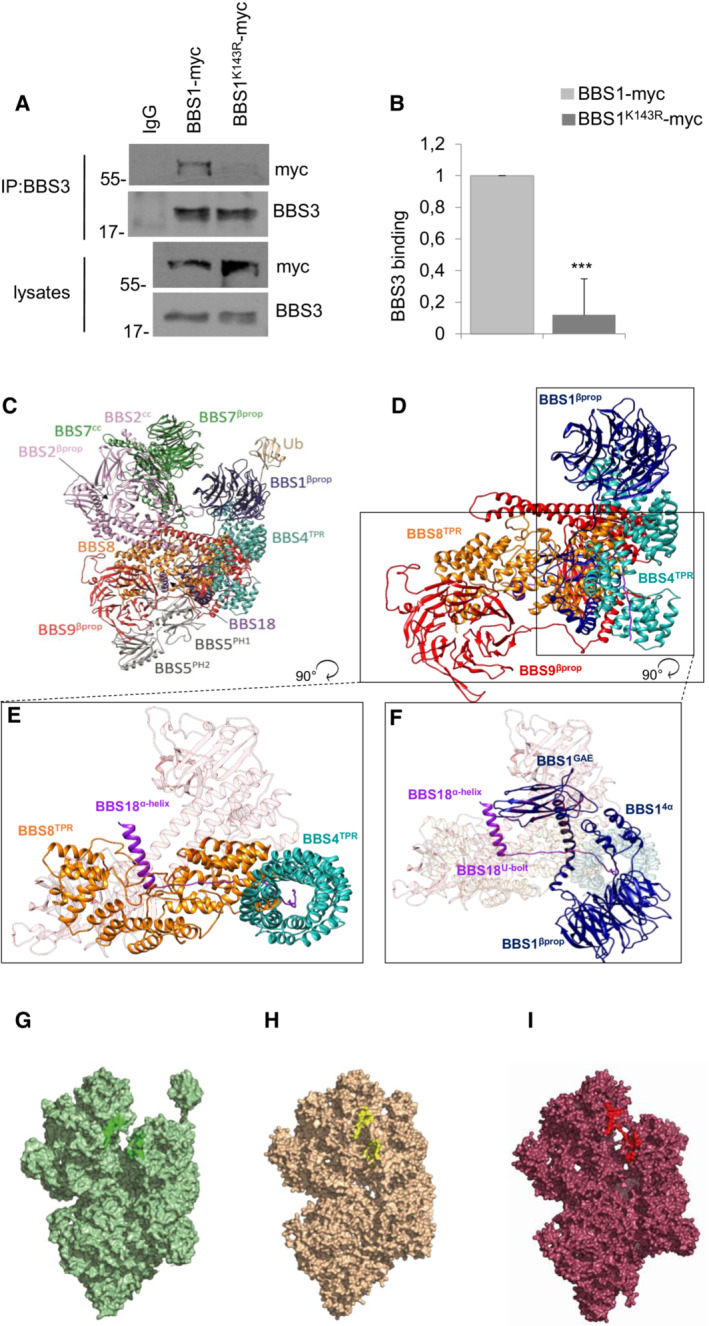
A
Co‐immunoprecipitation assay of BBS1 or BBS1K143R and BBS3 from lysates of HEK293 transiently expressing the indicated transgenes.
-
B
Cumulative data of the experiments shown in (A). The data are expressed as mean ± SD of three independent experiments. Student's t test, ***P < 0.001.
-
C
Full atomistic description of the homology model of the octameric complex of the BBSome human sequence (hBBSome) in the Arl6GTP‐bound (open) conformation, with the monoubiquitylation at K143 of hBBS14α (Ub‐hBBSome).
-
D
Focus on the full atomistic model of hBBSome core complex components (BBS1, BBS4, BBS8, BBS9, and BBS18).
-
E
Top view of the interaction details between BBS18 α‐helix and the BBS18 U‐bolt domains with the BBS8TPR and the superhelix of BBS4TPR, respectively.
-
F
Top view of the interaction details between BBS18 α‐helix with the BBS1GAE domain and the superhelix of BBS4TPR.
-
G
Surface representation of the CG‐MD model of the Ub‐hBBSome, representing the starting point of the CG‐MD simulation. Residues involved in the Arl6GTP‐bound binding mode are highlighted in dark green.
-
H, I
Surface representation of the last CG‐MD frame of the wt‐hBBSome and the Ub‐hBBSome, respectively showing the progressive closure of Arl6‐binding site located between BBS1βprop and BBS7βprop. Residues involved in the Arl6GTP‐bound binding mode are highlighted in red and yellow, respectively.