Abstract
Introduction
The efficacy and safety of atezolizumab in previously treated patients with NSCLC have been established in the registrational phase 3 OAK trial. In this study, we evaluated the effectiveness and safety of atezolizumab monotherapy in a large real-world cohort to confirm the reproducibility of the results of the registrational trial.
Methods
This was a multicenter, prospective, single-arm observational study. Consecutive patients with previously treated NSCLC scheduled to receive atezolizumab monotherapy were enrolled. The primary end point was the 18-month overall survival (OS) rate. The incidence of adverse events (AEs) and immune-related AEs was evaluated.
Results
Overall, 1002 patients were included in the safety analysis set and 1000 in the full analysis set. Median follow-up was 11.5 months. Of the full analysis set, 62% were ineligible for the OAK trial (OAK-unlike subpopulation). The 18-month OS rate was 41.1%, with a median OS of 13.0 months (95% confidence interval: 12.2–15.1). The 18-month OS rate was 49.4% and 36.1% in OAK-like and OAK-unlike subpopulations, respectively; that in patients with Eastern Cooperative Oncology Group performance status greater than or equal to 2 was 14.3%. The incidence of AEs overall, in the OAK-like, and OAK-unlike subpopulations was 43.9%, 46.2%, and 42.5%; that of immune-related AEs was 19.0%, 20.1%, and 18.3%, respectively.
Conclusions
The findings suggest that atezolizumab may be effective and safe for previously treated patients with NSCLC in real-world settings; however, atezolizumab administration should be considered carefully regarding the benefit–risk balance for the OAK-unlike subpopulation, especially in patients with Eastern Cooperative Oncology Group performance status greater than or equal to 2.
Keywords: Non–small cell lung cancer, Immune checkpoint inhibitors, Atezolizumab, Observational study
Introduction
Until the advent of immunotherapy, the 5-year survival rate of patients with advanced NSCLC treated with chemotherapy was less than 5%.1 When cancer spreads from the lung to distant parts of the body, it is advanced and uncurable and is known as metastatic NSCLC. Effective treatments for metastatic NSCLC are limited, especially for patients without targetable oncogene driver mutations. Immune checkpoint inhibitors (ICIs) were found to have clinically meaningful survival benefits, durable responses, and favorable safety profiles versus chemotherapy and are treatment options for patients with advanced NSCLC.2, 3, 4, 5, 6 Therefore, the treatment landscape for patients with advanced NSCLC is gradually improving.
Atezolizumab is a recombinant humanized monoclonal antibody of the immunoglobulin G1 subclass against human programmed death-ligand 1 (PD-L1) that targets PD-L1 expressed in tumor or immune cells. The OAK trial was the first randomized, open-label, global phase 3 study to evaluate the efficacy and safety of PD-L1–targeted therapy with atezolizumab versus docetaxel (standard of care) in previously treated patients with NSCLC.5 The results revealed a statistically and clinically significant improvement in overall survival (OS) with atezolizumab versus docetaxel in this patient population, regardless of PD-L1 expression or histology (squamous or nonsquamous), with a favorable safety profile compared with docetaxel (median OS; 13.8 months [95% confidence interval (CI), 11.8–15.7] versus 9.6 months [95% CI, 8.6–11.2]; hazard ratio [HR], 0.73 [95% CI, 0.62–0.87]; p = 0.0003).
Because of strict eligibility criteria, patients enrolled in clinical trials do not typically have the same characteristics as those encountered in real-world settings. The OAK trial, similar to other clinical trials, did not enroll patients with Eastern Cooperative Oncology Group performance status (ECOG PS) 2 to 4, patients with untreated central nervous system (CNS) metastasis, those with autoimmune disease, and those who had three or more previous treatment regimens.5 Furthermore, detailed data regarding immune-related adverse events (irAEs) have not been reported. Therefore, we considered it important to determine whether the results of clinical trials can be reproduced in terms of effectiveness and frequency of toxicity, including problematic irAEs, among patients in a real-world setting.
A global phase 3 and 4 study, TAIL (NCT03285763), confirmed the benefit–risk profile of atezolizumab monotherapy in a clinically diverse population of patients with previously treated NSCLC.7 In this study, patients ineligible for registrational trials accounted for approximately 66%, confirming the landmark survival rate at 12 months.
On this basis, the current study aimed to evaluate the reproducibility of the effectiveness and safety of atezolizumab monotherapy in a large sample of real-world Japanese patients with unresectable advanced or recurrent NSCLC, considering the eligibility criteria of the OAK trial.
Materials and Methods
Study Design and Treatment
The present study was a multicenter, noninterventional, nonblinded, single-arm, prospective observational study. Consecutive patients scheduled to receive atezolizumab monotherapy were enrolled from August 15, 2018, to October 16, 2019, at 197 institutions in Japan.
Each patient received atezolizumab monotherapy according to the latest package insert.8 Decisions on dose interruptions or withdrawals of atezolizumab were made at the physician’s discretion in accordance with the atezolizumab package insert and the Guidelines for the Promotion of Optimal Use.9
The present study was conducted in accordance with the Declaration of Helsinki, Ethical Guidelines for Medical and Health Research Involving Human Subjects, and the International Council for Harmonisation guidelines for Good Clinical Practice. All patients provided written informed consent for study participation. This study was registered at UMIN-CTR under the identifier number UMIN000033133 and at ClinicalTrials.gov under the identifier number NCT03645330.
Patients
Details of eligibility criteria are found in Supplementary Table 1. Patients who were scheduled to receive atezolizumab monotherapy and met the following enrollment criteria were included: aged above or equal to 20 years at the time of informed consent, diagnosed with having unresectable advanced or recurrent NSCLC, and previously treated with systemic therapy. Patients were excluded if they were considered unsuitable for enrollment by the investigator.
Effectiveness End Points
The primary end point was the OS rate at 18 months. Secondary end points were median OS; OS rate at 12 and 24 months; median progression-free survival (PFS); PFS rates at 6, 12, 18, and 24 months; time-to-treatment failure (TTF); TTF rates at 6, 12, 18, and 24 months; objective response rate (ORR); duration of response (DOR); and disease control rate (DCR). The investigators assessed progression and response according to Response Evaluation Criteria in Solid Tumors version 1.1, without confirmatory measurement.10
Safety End Points
The frequency of adverse events (AEs) and immune-related AEs (irAEs) was tabulated according to grade and severity assessed by the investigators on the basis of the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.11
Subgroup Analysis
The effectiveness and safety analyses were conducted in patient subgroups according to baseline characteristics, including CNS metastasis at the time of registration, age above or equal to 75 years, ECOG PS greater than or equal to 2, previous treatment with ICIs, history of autoimmune disease, and targetable driver oncogene status (EGFR mutation status, ALK rearrangement status, ROS1 rearrangement status, and BRAF V600E mutation status).
Considering the differences between patients enrolled in clinical trials and those from real-world settings, the effectiveness end points were evaluated in OAK-like and OAK-unlike subpopulations. Patients who met the following criteria were classified as the OAK-unlike subpopulation: those with ECOG PS 2 to 4, previous treatment with ICIs, CNS metastases at baseline, creatine clearance less than 30 mL/min, liver impairment, history of autoimmune disease, with three or more previous treatment regimens, and had not received previous platinum combination therapy. Patients with missing data on these criteria were classified as the OAK-unlike population. Other than the OAK-unlike subpopulation, all patients were classified as the OAK-like subpopulation. In addition, a subgroup analysis by PD-L1 expression (tumor proportion score [TPS] <1%, 1%–49%, ≥50%), using PD-L1 22C3 immunohistochemistry assay, was performed in OAK-like and OAK-unlike subpopulations.
Patient-Reported Outcomes
Patient-reported outcomes (PROs) were evaluated using the Japanese version of the European Quality of Life Five-Dimension Five-Level (EQ-5D-5L) questionnaire.12,13 This questionnaire measures the following five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Higher scores represent better quality of life. A worsening symptom was defined as a score reduction of 0.05 or 0.1 from baseline.
Statistical Methods
There was no formal statistical hypothesis linked to the sample size calculation. The planned sample size was 1000 patients, which was considered sufficient to estimate a 95% CI within a range of 20% for the 18-month OS rate of the subpopulation of clinical interest.
The safety analysis set was used to assess safety, and the full analysis set (FAS) was used as the primary analysis population to evaluate effectiveness. PROs were evaluated in the PRO analysis population. The safety analysis set included patients who received at least one dose of the study drug after enrollment. The FAS included all enrolled patients, excluding ineligible patients and those with no postdose effectiveness evaluation. The PRO analysis population included the FAS population who completed a baseline PRO survey and had at least one PRO survey assessment after baseline.
Descriptive statistics were used to summarize the baseline characteristics of patients, using median (interquartile range or range) for continuous variables and n (%) for categorical variables. The Kaplan-Meier method was used to estimate the OS rates at 12, 18, and 24 months and PFS and TTF rates at 6, 12, 18, and 24 months, and 95% CIs were calculated using the Greenwood formula. For secondary effectiveness end points (median OS, PFS, TTF, and DOR), a Kaplan-Meier curve was constructed to calculate the median time-to-event onset, and 95% CIs were calculated using the Brookmeyer–Crowley method. The HR was estimated with a stratified Cox regression analysis to compare the OAK-like and -unlike subpopulations. The proportion of patients with complete response (CR) or partial response (PR) was calculated for ORR. For DCR, the proportion of patients with CR, PR, or stable disease (SD) maintaining for more than 24 weeks was calculated; a normal approximation was used to calculate the 95% CIs. No imputation method was used for missing data. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patients
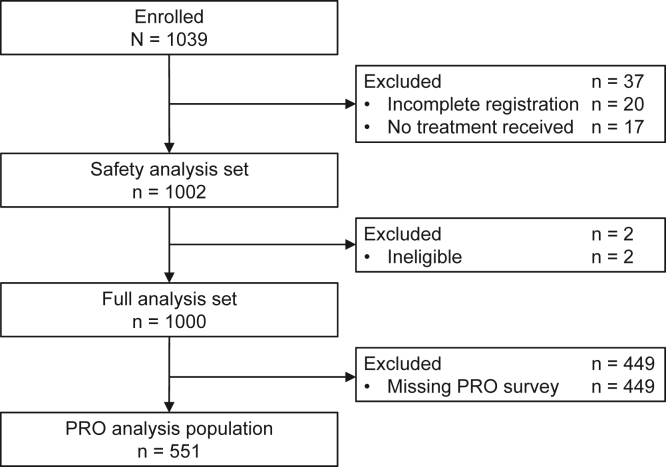
A total of 1039 patients were enrolled between August 2018 and October 2019 at 169 sites in Japan. Among them, 1002 were included in the safety analysis set, and 1000 were included in the FAS (Fig. 1). The median follow-up was 11.5 months (interquartile range, 4.3–20.3) in the FAS.
Figure 1.
Patient disposition. PRO, patient-reported outcome.
Patient baseline demographic and clinical characteristics are found in Table 1. In the FAS, 289 (28.9%) patients were above or equal to 75 years old, 120 (12.0%) patients had an ECOG PS greater than or equal to 2, 20 (2.0%) patients had a history of autoimmune disease, 191 (19.1%) patients had CNS metastases, 355 (35.5%) patients had received atezolizumab as more than or equal to fourth-line treatment, and 219 (21.9%) patients had previous treatment with ICIs. EGFR, ALK, ROS1, and BRAF targetable driver gene alterations were present in 146 (14.6%), five (0.5%), three (0.3%), and one (0.1%) patient(s), respectively. Of the PD-L1 assays, the most frequent were PD-L1 22C3 immunohistochemistry assay (80.1%, n = 801), and patients with TPS less than 1% (44.4%, n = 356) were the most common. Details of PD-L1 assays are found in Supplementary Table 2. The OAK-like subpopulation comprised 37.9% of the FAS (n = 379). Patient baseline demographic and clinical characteristics in the OAK-like and -unlike subpopulations are found in Supplementary Table 3.
Table 1.
Patient Background Demographic and Clinical Characteristics (Full Analysis Set)
| Characteristics | Full Analysis Set N = 1000 |
|---|---|
| Sex | |
| Male | 718 (71.8) |
| Female | 282 (28.2) |
| Age, y | |
| Median age (range) | 71 (34–93) |
| ≥75 | 289 (28.9) |
| Histology | |
| Squamous | 216 (21.6) |
| Nonsquamous | 737 (73.7) |
| Other | 47 (4.7) |
| ECOG PS | |
| 0 | 335 (33.5) |
| 1 | 545 (54.5) |
| 2 | 107 (10.7) |
| 3 | 13 (1.3) |
| 4 | 0 (0) |
| Smoking history | 756 (75.6) |
| Medical history | |
| Autoimmune disease | 20 (2.0) |
| Other than autoimmune disease | 537 (53.7) |
| Complications | |
| Autoimmune disease | 68 (6.8) |
| Other than autoimmune disease | 708 (70.8) |
| Primary tumor surgery | 286 (28.6) |
| Metastases | |
| CNS | 191 (19.1) |
| Bone | 263 (26.3) |
| Adrenal | 91 (9.1) |
| Liver | 126 (12.6) |
| Kidney | 13 (1.3) |
| Othera | 806 (80.6) |
| Stage | |
| IIIA | 42 (4.2) |
| IIIB | 60 (6.0) |
| IIIC | 14 (1.4) |
| IVA | 284 (28.4) |
| IVB | 311 (31.1) |
| Postsurgery recurrence | 211 (21.1) |
| Postchemoradiation therapy recurrence | 78 (7.8) |
| Treatment line of atezolizumab | |
| 2 | 425 (42.5) |
| 3 | 220 (22.0) |
| ≥4 | 355 (35.5) |
| Prior drug therapy | |
| Immune checkpoint inhibitors | 219 (21.9) |
| Chemotherapy | 988 (98.8) |
| Angiogenesis inhibitor | 388 (38.8) |
| EGFR inhibitor | 156 (15.6) |
| ALK inhibitor | 7 (0.7) |
| Other | 7 (0.7) |
| Prior radiation therapy | 288 (28.8) |
| Targetable driver oncogene status | |
| Negativeb | 128 (12.8) |
| Positiveb | 155 (15.5) |
| Unknownb | 593 (59.3) |
| EGFR mutation status | |
| Positive | 146 (14.6) |
| ALK rearrangement status | |
| Positive | 5 (0.5) |
| ROS1 rearrangement status | |
| Positive | 3 (0.3) |
| BRAF V600E mutation status | |
| Positive | 1 (0.1) |
| PD-L1 | |
| 22C3 | |
| n | 801 |
| TPS ≥50% | 138 (17.2) |
| TPS 1%–49% | 307 (38.3) |
| TPS <1% | 356 (44.4) |
| Additional predefined key subgroups | |
| OAK-like population | 379 (37.9) |
| OAK-unlike population | 621 (62.1) |
| Renal impairmentc | 25 (2.5) |
| Liver impairmentc | 51 (5.1) |
CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; ICIs, immune checkpoint inhibitors; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
Other includes patients with pleural effusion.
Negative, all targetable driver oncogene statuses (EGFR mutation status, ALK rearrangement status, ROS1 rearrangement status, and BRAF V600E mutation status) were negative; positive, one or more were positive; unknown, none of the positives, any were unknown or not tested.
Based on baseline laboratory values, any missing values were treated as impairment.
Effectiveness in the FAS Population
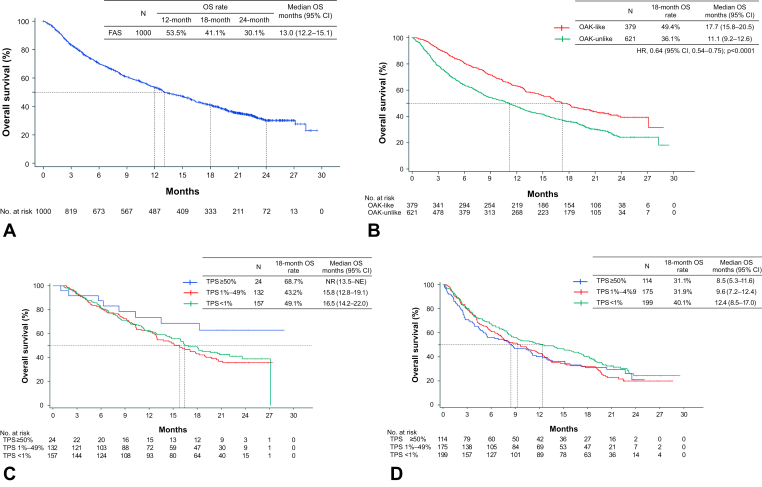
Effectiveness end points in the FAS are summarized in Table 2. The OS rate at 18 months (primary effectiveness end point) was 41.1% (95% CI: 38.0–44.3) in the FAS. Regarding secondary effectiveness end points, the median OS was 13.0 months (95% CI: 12.2–15.1) (Fig. 2A), the median PFS was 2.1 months (95% CI: 2.1–2.3) (Supplementary Fig. 1A), and the median TTF was 2.1 months (95% CI: 2.0–2.1) (Supplementary Fig. 1B).
Table 2.
Effectiveness in All Patients and Selected Subgroups
| End Points | Overall | OAK-Like Population | OAK-Unlike Population | CNS Metastases | ECOG PS ≥ 2 | Previous Treatment With ICIs | Autoimmune Disease | Age ≥ 75 y | |
|---|---|---|---|---|---|---|---|---|---|
| N | 1000 | 379 | 621 | 191 | 120 | 219 | 20 | 289 | |
| OS events | 622 | 205 | 417 | 142 | 100 | 156 | 14 | 184 | |
| Median OS, months (95% CI) | 13.0 (12.2–15.1) | 17.7 (15.8–20.5) | 11.1 (9.2–12.6) | 7.7 (6.0–10.7) | 2.8 (2.3–3.8) | 10.3 (8.1–12.2) | 8.6 (5.6–19.9) | 12.7 (9.6–14.6) | |
| 18-mo OS, % (95% CI) | 41.1 (38.0–44.3) | 49.4 (44.1–54.7) | 36.1 (32.1–40.0) | 27.4 (20.8–34.0) | 14.3 (7.6–21.0) | 29.9 (23.6–36.3) | 35.0 (14.1–55.9) | 36.0 (30.1–41.9) | |
| Median PFS, months (95% CI) | 2.1 (2.1–2.3) | 2.6 (2.2–3.0) | 2.1 (2.0–2.1) | 1.6 (1.4–1.9) | 1.2 (1.0–1.5) | 2.1 (1.8–2.2) | 2.6 (2.1–5.6) | 2.2 (2.1–2.6) | |
| BOR | n (%) | 979 | 377 | 602 | 181 | 114 | 213 | 20 | 283 |
| CR | 9 (0.9) | 3 (0.8) | 6 (1.0) | - | - | 2 (0.9) | - | 5 (1.8) | |
| PR | 77 (7.9) | 38 (10.1) | 39 (6.5) | 9 (5.0) | 4 (3.5) | 9 (4.2) | 1 (5.0) | 27 (9.5) | |
| SD | 311 (31.8) | 131 (34.7) | 180 (29.9) | 40 (22.1) | 22 (19.3) | 68 (31.9) | 7 (35.0) | 87 (30.7) | |
| PD | 470 (48.0) | 172 (45.6) | 298 (49.5) | 104 (57.5) | 51 (44.7) | 104 (48.8) | 9 (45.0) | 129 (45.6) | |
| NE | 112 (11.4) | 33 (8.8) | 79 (13.1) | 28 (15.5) | 37 (32.5) | 30 (14.1) | 3 (15.0) | 35 (12.4) | |
| ORR | n (%) | 86 (8.8) | 41 (10.9) | 45 (7.5) | 9 (5.0) | 4 (3.5) | 11 (5.2) | 1 (5.0) | 32 (11.3) |
| 95% CI | (7.0–10.6) | (7.7–14.0) | (5.4–9.6) | (1.8–8.1) | (0.1–6.9) | (2.2–8.1) | (0.0–14.6) | (7.6–15.0) | |
| DCR | n (%) | 145 (14.8) | 70 (18.6) | 75 (12.5) | 15 (8.3) | 7 (6.1) | 20 (9.4) | 2 (10.0) | 45 (15.9) |
| 95% CI | (12.6–17.0) | (14.6–22.5) | (9.8–15.1) | (4.3–12.3) | (1.7–10.5) | (5.5–13.3) | (0.0–23.1) | (11.6–20.2) | |
| Maintaining SD 24 wk, n (%) | 59 (6.0) | 29 (7.7) | 30 (5.0) | 6 (3.3) | 3 (2.6) | 9 (4.2) | 1 (5.0) | 13 (4.6) | |
BOR, best overall response; CI, confidence interval; CNS, central nervous system; CR, complete response; DCR, disease control rate; ECOG PS, Eastern Cooperative Oncology Group performance status; ICIs, immune checkpoint inhibitors; NE, not evaluable; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Figure 2.
Kaplan-Meier curves for OS. (A) FAS. (B) OAK-like and OAK-unlike subpopulations. (C) OAK-like subpopulation according to PD-L1 expression. (D) OAK-unlike subpopulation according to PD-L1 expression. CI, confidence interval; FAS, full analysis set; HR, hazard ratio; mo, months; NE, not estimable; NR, not reached; OS, overall survival; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
Among 979 patients with measurable lesions, the best overall response was CR in nine (0.9%) patients, PR in 77 (7.9%) patients, SD in 311 (31.8%) patients, and progressive disease in 470 (48.0%) patients (Table 2). The ORR was 8.8% (95% CI: 7.0–10.6), and DCR was 14.8% (95% CI: 12.6–17.0). The median DOR was 15.6 months (95% CI: 12.3–not evaluable [NE]) (Supplementary Fig. 1C).
Effectiveness in Subgroups
Patient baseline demographic and clinical characteristics according to subgroups are found in Supplementary Table 3. Effectiveness end points in the selected subgroups are summarized in Table 2. Results for subgroup analysis by targetable driver oncogene status are found in Supplementary Table 4. Kaplan-Meier curves for OS and PFS in the subgroups according to targetable driver oncogene status are found in Supplementary Figures 2A and B. The OS and PFS tended to be slightly poorer in mutation-positive patients than in -negative patients. The OAK-like and OAK-unlike subpopulations achieved an 18-month survival rate of 49.4% (95% CI: 44.1–54.7) and 36.1% (95% CI: 32.1–40.0), respectively. The median OS was 17.7 months (95% CI: 15.8–20.5) in the OAK-like subpopulation versus 11.1 months (95% CI: 9.2–12.6) in the OAK-unlike subpopulation (HR: 0.64; 95% CI: 0.54–0.75; p < 0.0001). The median PFS was 2.6 months (95% CI: 2.2–3.0) in the OAK-like subpopulation versus 2.1 months (95% CI: 2.0–2.1) in the OAK-unlike subpopulation (HR: 0.81; 95% CI: 0.71–0.93; p = 0.0022). Kaplan-Meier curves for OS and PFS in the OAK-like and OAK-unlike subpopulations are found in Figure 2B and Supplementary Figure 3A.
Kaplan-Meier curves for OS by PD-L1 expression status in the OAK-like and -unlike subpopulations are found in Figure 2C and D. In patients with TPS greater than or equal to 50%, the OS rate at 18 months was 68.7% and 31.1% in the OAK-like and -unlike subpopulations, respectively. In the OAK-like subpopulation, the median OS was not reached (95% CI: 13.5–NE) for TPS greater than or equal to 50%, 15.8 months (95% CI, 12.8–19.1) for TPS 1% to 49%, and 16.5 months (95% CI, 14.2–22.0) for TPS less than 1%. In the OAK-unlike subpopulation, the median OS was 8.5 months (95% CI, 5.3–11.6) for TPS greater than or equal to 50%, 9.6 months (95% CI, 7.2–12.4) for TPS 1% to 49%, and 12.4 months (95% CI: 8.5–17.0) for TPS less than 1%. The median PFS in the OAK-like subpopulation by PD-L1 expression status in the OAK-like subpopulation was 5.2 months (95% CI: 2.1–17.1) for TPS greater than or equal to 50%, 2.6 months (95% CI: 2.1–3.3) for TPS 1% to 49%, and 2.3 months (95% CI: 2.0–3.0) for TPS less than 1% (Supplementary Fig. 3B); and 2.0 months (95% CI: 1.6–2.7) for TPS greater than or equal to 50%, 2.0 months (95% CI: 1.6–2.1) for TPS 1% to 49%, and 2.1 months (95% CI: 1.9–2.3) for TPS less than 1% (Supplementary Fig. 3C).
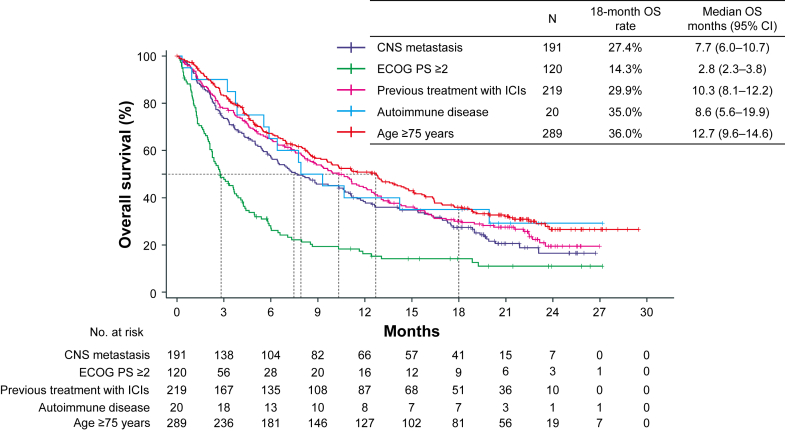
Kaplan-Meier curves for OS and 18-month OS rates in the OAK-unlike population with the selected subgroups are found in Figure 3 and Table 2. Kaplan-Meier curves for PFS in the OAK-unlike population with the selected subgroups are found in Supplementary Figure 4. Patients previously treated with ICIs had a relatively favorable median OS in this OAK-unlike subpopulation. Patients in the subgroups of CNS metastases, ECOG PS greater than or equal to 2, and a history of autoimmune disease had a generally poor prognosis (Fig. 3 and Table 2). The best overall response, ORR, and DCR in the selected subgroups are found in Table 2. Although the tumor response was equivalent among patients aged above or equal to 75 years (ORR, 11.3%; 95% CI: 7.6%–15.0%) and the overall population, tumor responses in other subgroups were limited.
Figure 3.
Kaplan-Meier curve for OS in the subgroups. CI, confidence interval; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; ICIs, immune checkpoint inhibitors; OS, overall survival.
Safety
The incidence of AEs was 43.9% (440 of 1002) and that of irAEs was 19.0% (190 of 1002) in the safety analysis set (Supplementary Table 5 and Table 3). The incidence of treatment-related AEs was 26.4% (265 of 1002); grade 3 or 4 treatment-related AEs, 8.7% (87 of 1002); serious AEs, 20.6% (206 of 1002); and treatment-related deaths, 1.4% (14 of 1002) (Supplementary Table 5). The incidence of treatment-related irAEs was 18.2% (182 of 1002); grade 3 to 4 treatment-related irAEs, 7.0% (70 of 1002); and irAEs leading to withdrawal from treatment, 5.8% (58 of 1002) (Table 3).
Table 3.
Summary of irAEs in the Overall Study Population and Patient Subgroups (Safety Analysis Set)
| irAEs | Overall | OAK-Like Population | OAK-Unlike Population | CNS Metastases | ECOG PS ≥ 2 | Previous Treatment With ICIs | Autoimmune Disease | Age ≥ 75 y |
|---|---|---|---|---|---|---|---|---|
| N | 1002 | 379 | 623 | 191 | 120 | 219 | 20 | 290 |
| All irAEs | 190 (19.0) | 76 (20.1) | 114 (18.3) | 31 (16.2) | 15 (12.5) | 34 (15.5) | 7 (35.0) | 58 (20.0) |
| Treatment-related irAEs | 182 (18.2) | 72 (19.0) | 110 (17.7) | 30 (15.7) | 15 (12.5) | 32 (14.6) | 7 (35.0) | 57 (19.7) |
| Grade 3 or 4 irAEs | 74 (7.4) | 28 (7.4) | 46 (7.4) | 15 (7.9) | 6 (5.0) | 14 (6.4) | 3 (15.0) | 21 (7.2) |
| Grade 3 or 4 treatment-related irAEs | 70 (7.0) | 26 (6.9) | 44 (7.1) | 14 (7.3) | 6 (5.0) | 12 (5.5) | 3 (15.0) | 20 (6.9) |
| All deaths | 12 (1.2) | 4 (1.1) | 8 (1.3) | 2 (1.0) | 2 (1.7) | 3 (1.4) | 0 (0) | 3 (1.0) |
| Treatment-related deaths | 12 (1.2) | 4 (1.1) | 8 (1.3) | 2 (1.0) | 2 (1.7) | 3 (1.4) | 0 (0) | 3 (1.0) |
| Serious irAEs | 87 (8.7) | 34 (9.0) | 53 (8.5) | 18 (9.4) | 9 (7.5) | 17 (7.8) | 1 (5.0) | 26 (9.0) |
| irAEs leading to withdrawal from treatment | 58 (5.8) | 21 (5.5) | 37 (5.9) | 7 (3.7) | 5 (4.2) | 13 (5.9) | 3 (15.0) | 13 (4.5) |
| irAEs leading to dose interruption | 71 (7.1) | 26 (6.9) | 45 (7.2) | 15 (7.9) | 4 (3.3) | 11 (5.0) | 3 (15.0) | 24 (8.3) |
CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event.
Safety in Subgroups
The incidence of AEs and irAEs in subgroups according to baseline characteristics is found in Supplementary Table 5 and Table 3. The incidence of AEs, irAEs, and irAEs leading to treatment discontinuation in patients with a history of autoimmune disease tended to be higher, at 60.0%, 35.0%, and 15.0%, respectively. The incidence of AEs and irAEs in the other subgroups was consistent.
Patient-Reported Outcomes
Kaplan-Meier curves for time to worsening symptoms with a 0.05 and 0.1 reduction from baseline in EQ-5D-5L are found in Supplementary Figure 5A and B.
Discussion
In the present study, we evaluated the real-world effectiveness and safety of atezolizumab monotherapy in a very large number of patients with unresectable advanced or recurrent NSCLC. The present study enrolled more than 1000 patients to evaluate diverse characteristics in the real-world setting with a relatively long follow-up, including those not eligible for the registrational trial, and provided clinically meaningful data, including effectiveness and safety in older patients with poor prognosis as often encountered in real-world settings. Atezolizumab monotherapy yielded expected survival rates without new safety signals in patients with previously treated NSCLC, which were comparable with that of the registrational trial.
The primary end point of this study was the OS rate at 18 months in recurrent NSCLC patients treated with atezolizumab in a real-world setting. We set this primary end point to assess longer-term landmark survival data concerning the median survival time of 13.8 months reported in the registrational phase 3 OAK trial.5 In the present study, atezolizumab monotherapy was found to have comparable effectiveness with the OAK trial despite including patients excluded from the OAK trial (OAK-unlike subpopulation). The OS rate at 18 months was 41.1% in the FAS of the present study (median OS, 13.0 mo [95% CI: 12.2–15.1]) with a median follow-up of 11.5 months. These data were comparable with the atezolizumab arm of the OAK trial (18-mo OS rate, 40%; median OS, 13.8 mo [95% CI: 11.8–15.7]) with a median follow-up of 21.0 months.5 In this study, the median follow-up was immature for assessing the OS rate at 18 months. Further updates are needed to increase the robustness of the results.
In clinical practice, physicians often encounter patients whose characteristics do not match the eligibility criteria of registrational trials such as the OAK trial. Therefore, it is essential to understand the features of the OAK-unlike subpopulation. Atezolizumab monotherapy was found to have the benefit–risk profile in a clinically diverse population of patients with previously treated NSCLC, according to a recent report from the global phase 3 and 4 TAIL study.7 Subgroup data for the OAK-like population in the TAIL trial were comparable with the OAK trial (median OS 13.8 mo [95% CI: 11.8–15.7]), despite being conducted in a real-world setting. In contrast, the results of the OAK-unlike population were not revealed. In this study, the OAK-unlike subpopulation accounted for approximately 60% of the overall study population, which was higher than that in the TAIL study (34%). Although the clinical background of the OAK-unlike population is concerning, this study reveals the effectiveness and safety in such a population. Notably, patients with a history of ICI treatment and those with ECOG PS greater than or equal to 2 accounted for 21.9% and 12.0% of the present study, respectively. The median OS of 11.1 months in the OAK-unlike subpopulation was shorter compared with 17.7 months in the OAK-like subpopulation (HR: 0.64). In addition, patients aged above or equal to 75 years accounted for 28.9% of the study population. Although there is still debate on how aging affects the efficacy of ICIs, previous studies, including the TAIL study, revealed that ICIs seem to be effective in patients aged above or equal to 75 years.7,14,15 As in previous reports, ICIs were found to be effective regardless of age in the relatively large number of real-world patients in this study, supporting the notion that ICI therapy may be an important treatment option for older patients. For clinical practice, the present data should be interpreted with caution considering the registration study results and strongly suggest the importance of a real-world-based study.
PD-L1 expression status using the 22C3 antibody is an important biomarker for selecting the most suitable treatment regimen in our clinical practice. Regarding the analyses of PD-L1 expression in the OAK-like subpopulation, better trends in both OS and PFS were observed in the subgroup with TPS greater than or equal to 50% compared with subgroups with TPS 1% to 49% or less than 1% in the present study (Fig. 2C). This analysis strongly suggests that PD-L1 expression evaluation using the 22C3 antibody might be feasible and valuable as a predictive biomarker of the effectiveness of atezolizumab therapy for real-world patients who match the eligibility for the registration trials. In addition, the efficacy of atezolizumab on PD-L1–negative patients found in the OAK study was reproduced in this real-world population analysis. Conversely, in the OAK-unlike subpopulation, OS and PFS were similar irrespective of PD-L1 expression level. The OAK-unlike subpopulation was heterogeneous and included patients with various background factors, such as patients who had received steroid therapy for CNS metastasis, those previously treated with ICIs, or those with complex comorbidities. These individual factors were very diverse, and it was not easy to discern or analyze these factors in each case. This is one of the limitations of this real-world study. Nevertheless, these results highlight the vague use of ICIs which relies on PD-L1 expression among high-risk patients who do not qualify for enrollment in registration trials.
In the present study, the incidence of AEs and treatment-related AEs was 43.9% and 26.4%, respectively. The incidence of these AEs was less than half that in the atezolizumab arm of the OAK trial (94% and 64%, respectively).5 AEs of less severity are generally not as well recorded in the real world as in clinical trials, which could explain the lower incidence of these AEs in our study compared with the OAK trial. The toxicity profiles of irAEs associated with atezolizumab were reported in the TAIL study, and the incidence of irAEs in the overall population was 9.6%.7 In the present study, the incidence of irAEs was 19.0%. There is a difference in the definition of irAEs between the TAIL study (AEs of special interest requiring corticosteroid treatment within 30 d of onset) and the present study (AEs judged to be irAEs by the investigator); the difference in the incidence may be because of this variation in definition. Nevertheless, the percentages of serious irAEs, irAEs leading to treatment discontinuation, and treatment-related death were 8.7%, 5.8%, and 1.2%, respectively. Despite the relatively high frequency of irAEs, the lower incidence of irAEs leading to treatment discontinuation and treatment-related death in our study suggests that atezolizumab treatment was well managed under actual clinical conditions with adequate caution for serious irAEs.
In the subgroup analysis, age did not affect the effectiveness of atezolizumab. Older age has been considered a negative predictor in ICI therapy because of aging-related immunosenescence. Several reports, including the TAIL study, recently indicated that older age was not associated with survival outcomes and severe toxicities.16 The results of this study support the idea that age should not be a deciding factor for prescribing ICIs. Similarly, a history of ICI therapy was not a negative predictor for survival outcomes. Nevertheless, this population’s response rate and median PFS were 5.2% and 2.1 months, respectively (Table 2). The use of atezolizumab beyond progression may provide favorable disease control in the post hoc analysis of the OAK trial.17 Atezolizumab may be a suitable treatment option in this setting, but physicians should consider the benefit–risk balance carefully, including the economic burden. The present study revealed that the incidence of irAEs tended to be higher among patients with autoimmune disease than in other patient subgroups. This trend was similar to that found in previous reports.7,18 In terms of effectiveness, although the median OS in patients with an autoimmune disease in the present study was short at 8.6 months, their OS rate at 18 months (35.0%) was comparable with that of the OAK trial at 18 months (40.0%). These findings suggest that careful attention should be given to the onset of irAEs; however, treatment should not be delayed owing to autoimmune disease complications alone. Long-term survivors were generally recognized even among patients with several characteristics that led to the ineligibility for the OAK trial, such as CNS metastasis and a history of autoimmune disease. For the OAK-unlike population, atezolizumab may be a reasonable therapy option with careful consideration of the benefit–risk balance; however, effectiveness outcomes were unfavorable for patients with ECOG PS greater than or equal to 2, consistent with previous studies. This strongly suggests that poor PS was a negative predictive and prognostic factor of immunotherapy with PD-1 or PD-L1 inhibitors.19,20 Although no safety concerns were observed in this study regarding the poor PS population, the benefits were limited. Thus, ICI therapy for this population should be considered with great caution.
The present study has some limitations. First, this study has an observational design. Owing to the study’s observational nature, there was no control group. Moreover, the progression measurement was susceptible to error, and the frequency of radiographic examinations was uneven across participating sites for PFS evaluation. Second, the median observation period of this study was 11.5 months, which was not sufficient to evaluate the “long-tail effect.” Although the adequate observation period to evaluate the so-called long-tail effect has not been established, it is considered that an observation period of more than 5 years has been required empirically. Future observation and updates of survival and longer-term safety data are needed. Third, this study was conducted only in Japan and results are based only on the Japanese population. Nevertheless, although studies on the effect of ICI monotherapy for NSCLC with driver mutations including EGFR mutation are limited, this study analyzed a large number of patients (n = 146) and found a slightly inferior trend in positivity, as in previous reports. The OS and PFS tended to be slightly poorer in mutation-positive patients than in -negative patients, in line with a previous report.21 Prospective studies, including the ongoing chemotherapy plus ICI study, will reveal the use of ICIs in NSCLC with driver mutations.
In conclusion, the results of the J-TAIL study revealed comparable effectiveness of atezolizumab to the registrational phase 3 OAK trial. In addition, no new safety signals were identified. An acceptable benefit–risk profile was observed among patients who would have been ineligible for the OAK trial. Nevertheless, the administration of atezolizumab should be considered carefully regarding its effectiveness for patients with ECOG PS greater than or equal to 2, along with its safety in those with a history of autoimmune disease. In clinical practice, PD-L1 expression level may not predict effectiveness in patients who are ineligible for clinical trials.
CRediT Authorship Contribution Statement
Satoru Miura: Conceptualization, Resources, Investigation, Visualization, Writing—original draft, Writing—review and editing.
Makoto Nishio: Conceptualization, Resources, Supervision, Investigation, Writing—review and editing.
Hiroaki Akamatsu: Conceptualization, Resources, Investigation, Writing—review and editing.
Yasushi Goto: Conceptualization, Resources, Investigation, Writing—review and editing.
Hidetoshi Hayashi: Conceptualization, Resources, Investigation, Writing—review and editing.
Akihiko Gemma: Conceptualization, Resources, Supervision, Funding acquisition, Investigation, Writing—review and editing.
Ichiro Yoshino: Conceptualization, Resources, Investigation, Writing—review and editing.
Toshihiro Misumi: Formal analysis, Writing—review and editing.
Akito Hata: Resources, Investigation, Writing—review and editing.
Osamu Hataji: Resources, Investigation, Writing—review and editing.
Kohei Fujita: Resources, Investigation, Writing—review and editing.
Masahiro Seike: Resources, Investigation, Writing—review and editing.
Noriko Yanagitani: Resources, Investigation, Writing—review and editing
Kazumi Nishino: Resources, Investigation, Writing—review and editing.
Satoshi Hara: Resources, Investigation, Writing—review and editing.
Ryota Saito: Resources, Investigation, Writing—review and editing.
Masahide Mori: Resources, Investigation, Writing—review and editing.
Takeshi Tsuda: Resources, Investigation, Writing—review and editing.
Shunichiro Iwasawa: Conceptualization, Visualization, Writing—original draft, Project administration, Writing—review and editing.
Shintaro Nakagawa: Formal analysis, Writing—review and editing.
Tetsuya Mitsudomi: Conceptualization, Resources, Supervision, Funding acquisition, Investigation, Visualization, Project administration, Writing—review and editing.
Acknowledgments
The authors thank the patients, their families, the investigators, and the clinical study sites for participating in this study. We express our gratitude to the late Dr. Koichi Nakatani and the late Dr. Toru Kumagai for their contributions to this study. We thank Michelle Belanger, MD, of Edanz (www.edanz.com), for providing medical writing support, funded by Chugai Pharmaceutical Co., Ltd., and the staff members of Mebix, Inc., for their assistance with the study management, data collection, storage, statistical analysis, and medical writing support funded by Chugai Pharmaceutical Co., Ltd. This study was funded by Chugai Pharmaceutical Co., Ltd., which jointly conducted this study with the Japanese Lung Cancer Association and collaborated with the academic authors on the study design, data collection, analysis, and interpretation. The authors prepared all manuscript drafts with editorial assistance funded by Chugai Pharmaceutical Co., Ltd. All authors approved the submission.
Data Availability
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://www.clinicalstudydatarequest.com/Default.aspx). For further details on the data-sharing policy of Chugai Pharmaceutical Co., Ltd., and how to request access to related clinical study documents, see here: www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html.
Footnotes
Disclosure: Dr. Miura reports receiving honoraria from Chugai Pharma, Taiho Pharma, Eli Lilly, Boehringer Ingelheim, Ono Pharma, AstraZeneca, Merck Sharp & Dohme, and Bristol-Myers Squibb. Dr. Nishio reports receiving payment/honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Sankyo Healthcare, Taiho Pharmaceutical, Merck Serono, and Astellas. Dr. Akamatsu reports receiving research funding from Amgen, Chugai Pharmaceutical Co., Ltd., and Merck Sharp & Dohme K.K.; receiving honoraria from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Merck Sharp & Dohme K.K., Nippon Kayaku Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Pfizer Inc., and Taiho Pharmaceutical Co., Ltd.; and having an advisory board membership for Amgen, Inc., and Janssen Pharmaceutical K.K. Dr. Goto reports receiving grants or contracts to the clinical trial group from AstraZeneca K.K. and Pfizer; receiving grants or contracts to own institution from AbbVie, Eli Lilly, Pfizer, Bristol-Myers Squibb, Ono, Novartis, Kyorin, Daiichi Sankyo, Novartis, and Preferred Network; receiving payment/honoraria from Eli Lilly, Chugai, Taiho, Boehringer Ingelheim, Ono, Bristol-Myers Squibb, Pfizer, Merck Sharp & Dohme, Novartis, Merck, and Thermo Fisher; having participation on a data safety monitoring board or advisory board for AstraZeneca, Chugai, Boehringer Ingelheim, Eli Lilly, Taiho, Pfizer, Novartis, Guardant Health Inc., Illumina, Daiichi Sankyo, Ono Pharmaceutical, Bristol-Myers Squibb, and Merck Sharp & Dohme; and having a leadership or fiduciary role in other board, society, committee, or advocacy group for Cancer Net Japan and JAMT. Dr. Hayashi reports receiving grants or contracts from AstraZeneca K.K., Astellas Pharma Inc., Merck Sharp & Dohme K.K., Ono Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Bristol-Myers Squibb Company, Eli Lilly Japan K.K., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Merck Serono Co., Ltd./Merck Biopharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., SymBio Pharmaceuticals Limited, AbbVie Inc., inVentiv Health Japan, ICON Japan K.K., GRITSONE ONCOLOGY Inc., PAREXEL International Corp., Kissei Pharmaceutical Co., Ltd., EPS Corporation, Syneos Health, Pfizer R&D Japan G.K., A2 Healthcare Corp., Quintiles Inc./IQVIA Services Japan K.K., EP-CRSU Co., Ltd., Linical Co., Ltd., Eisai Co., Ltd., CMIC Shift Zero K.K., Kyowa Hakko Kirin Co., Ltd., Bayer Yakuhin Ltd., EPS International Co., Ltd., and Otsuka Pharmaceutical Co., Ltd.; and payment/honoraria from Amgen K.K., AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol-Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly Japan K.K., Guardant Healthcare, Kyorin Pharmaceutical Co., Ltd., Merck Biopharma Co., Ltd., Merck Sharp & Dohme K.K., Novartis Pharmaceuticals K.K., Ono Pharmaceutical Co., Ltd., Shanghai Haihe Biopharm, Taiho Pharmaceutical Co., Ltd., Pfizer, and Takeda Pharmaceutical Co., Ltd. Dr. Gemma reports receiving lecture fees from Chugai Pharmaceutical Co., Ltd. Dr. Yoshino reports receiving lecture fees/advisory fees from Chugai Pharmaceutical, Intuitive Surgical, AstraZeneca, Johnson & Johnson, and Covidien Japan; grants/lecture fees from Taiho Pharmaceutical and Daiichi Sankyo Chemical Pharma; lecture fees from Japan Blood Products Organization, Astellas, Bristol-Meyers Squibb, Shionogi Pharmaceutical, Eli Lilly, CSL Behring, Merck Sharp & Dohme, Ono Pharmaceutical, and Care Net; advisory fee from Medicaroid; and grants from Pfizer. Dr. Misumi reports receiving grants or contracts to own institution from Chugai Pharmaceutical Co., Ltd. Dr. Hata reports receiving grants or contracts from Merck Sharp & Dohme, Eli Lilly, Boehringer Ingelheim, and AstraZeneca; and payment/honoraria from Eli Lilly, Chugai, Pfizer, AstraZeneca, and Boehringer Ingelheim. Dr. Hataji reports receiving grants or contracts from AstraZeneca, Novartis Pharma, Merck Sharp & Dohme, Daiichi Sankyo Company, Limited, Bayer Yakuhin, AbbVie, Eli Lilly, Janssen Pharmaceutical K.K, and Ono Pharmaceutical; and payment/honoraria from AstraZeneca, Chugai Pharmaceutical, Takeda Pharmaceutical, Merck Sharp & Dohme, Merck KGaA, Daiichi Sankyo Company Limited, Eli Lilly, AbbVie, Boehringer Ingelheim, Nippon Kayaku, and Taiho Pharmaceutical. Dr. Seike reports receiving research funding from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical, Merck Sharp & Dohme K.K., Nippon Boehringer Ingelheim, and Eli Lilly; and honoraria for lectures from Chugai Pharmaceutical Co., Ltd., AstraZeneca, Merck Sharp & Dohme K.K., Taiho Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Kyowa Hakko Kirin, Daiichi Sankyo Company, Novartis, and Nippon Kayaku. Dr. Yanagitani reports receiving payment/honoraria from Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Pfizer Inc., Eli Lilly and Company, AstraZeneca plc, Takeda Pharmaceutical Company Limited, and Bayer Yakuhin Ltd.; and payment for expert testimony from Chugai Pharmaceutical Co., Ltd. Dr. Nishino reports receiving grants or contracts to own institution from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Merck Sharp & Dohme, AbbVie, Daiichi Sankyo Company Limited, Amgen, Eisai Co., Ltd., Eli Lilly Japan, Nippon Boehringer Ingelheim, Pfizer, Takeda Pharmaceutical Company Limited, Merck, Janssen Pharmaceutical K.K., and AstraZeneca; and honoraria for lectures from Eli Lilly Japan, Nippon Boehringer Ingelheim, Novartis, Pfizer, Takeda Pharmaceutical Company Limited, Merck, Janssen Pharmaceutical K.K., Bristol-Myers Squibb, Nippon Kayaku, and AstraZeneca. Dr. Mori reports receiving research funding from Chugai, Ono, Merck Sharp & Dohme, and Delta-fly; and lecture fees from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Eli Lilly, Novartis, Chugai, Taiho, Kyowa-Kirin, Ono Pharmaceutical, Otsuka, Nihon-Kayaku, Pfizer, Daiichi Sankyo, Takeda, and Shionogi. Dr. Tsuda reports receiving honoraria from Chugai Pharmaceutical Co., Ltd. Dr. Iwasawa reports receiving honoraria for lectures from Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb Company, Ono Pharmaceutical Co., Ltd., and AstraZeneca K.K; and is an employee of Chugai Pharmaceutical Co., Ltd. Nakagawa is an employee of Chugai Pharmaceutical Co., Ltd. Dr. Mitsudomi reports receiving grants or contracts from Ono Pharmaceutical, Chugai Pharmaceutical, AstraZeneca, and Merck Sharp & Dohme; consulting fees from Ono Pharmaceutical, Bristol-Myers Squibb, Merck Sharp & Dohme, Chugai, and AstraZeneca; and payment/honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Merck Sharp & Dohme, Chugai, and AstraZeneca. The remaining authors declare no conflict of interest.
Cite this article as: Miura S, Nishio M, Akamatsu H, et al. Effectiveness and safety of atezolizumab monotherapy in previously treated Japanese patients with unresectable advanced or recurrent NSCLC: a multicenter, prospective, observational study (J-TAIL). JTO Clin Res Rep. 2023;4:100484.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100484.
Supplementary Data
References
- 1.National Cancer Institute SEER Cancer Statistics Review 1975–2015. https://seer.cancer.gov/archive/csr/1975_2015/ Accessed 12 March 2023.
- 2.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 5.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assi H.I., Kamphorst A.O., Moukalled N.M., Ramalingam S.S. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer. 2018;124:248–261. doi: 10.1002/cncr.31105. [DOI] [PubMed] [Google Scholar]
- 7.Ardizzoni A., Azevedo S., Rubio-Viqueira B., et al. Primary results from TAIL: a global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atezolizumab (Tecentriq for intravenous infusion) package insert [In Japanese] https://pins.japic.or.jp/pdf/newPINS/00068280.pdf Accessed 12 March 2023.
- 9.Pharmaceutical and Medical Devices Agency Guidelines for the promotion of optimal use of atezolizumab [In Japanese] https://www.pmda.go.jp/files/000250403.pdf Accessed 12 March 2023.
- 10.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute Common terminology criteria for adverse events (CTCAE) https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 Accessed 12 March 2023.
- 12.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamst-Klaussen T., Lamu A.N., Chen G., Olsen J.A. Assessment of outcome measures for cost-utility analysis in depression: mapping depression scales onto the EQ-5D-5L. BJPsych Open. 2018;4:160–166. doi: 10.1192/bjo.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landre T., Des Guetz G., Chouahnia K., Fossey-Diaz V., Culine S. Immune checkpoint inhibitors for patients aged ≥75 years with advanced cancer in first- and second-line settings: a meta-analysis. Drugs Aging. 2020;37:747–754. doi: 10.1007/s40266-020-00788-5. [DOI] [PubMed] [Google Scholar]
- 15.Petrelli F., Inno A., Ghidini A., Gori S., Bersanelli M. Efficacy of immune checkpoint inhibitors in elderly patients aged ≥75 years. Cancer Immunol Immunother. 2021;70:1777–1780. doi: 10.1007/s00262-020-02779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosaki K., Saka H., Hosomi Y., et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. 2019;135:188–195. doi: 10.1016/j.lungcan.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Gandara D.R., von Pawel J., Mazieres J., et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK Study. J Thorac Oncol. 2018;13:1906–1918. doi: 10.1016/j.jtho.2018.08.2027. [DOI] [PubMed] [Google Scholar]
- 18.Haanen J., Ernstoff M.S., Wang Y., et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol. 2020;31:724–744. doi: 10.1016/j.annonc.2020.03.285. [DOI] [PubMed] [Google Scholar]
- 19.Facchinetti F., Mazzaschi G., Barbieri F., et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer. 2020;130:155–167. doi: 10.1016/j.ejca.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Dall’Olio F.G., Maggio I., Massucci M., Mollica V., Fragomeno B., Ardizzoni A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors—a systematic review and meta-analysis of real world data. Lung Cancer. 2020;145:95–104. doi: 10.1016/j.lungcan.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Mazieres J., Drilon A., Lusque A., et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://www.clinicalstudydatarequest.com/Default.aspx). For further details on the data-sharing policy of Chugai Pharmaceutical Co., Ltd., and how to request access to related clinical study documents, see here: www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html.