Scheme 3. Scalability and Diverse Transformationsa.
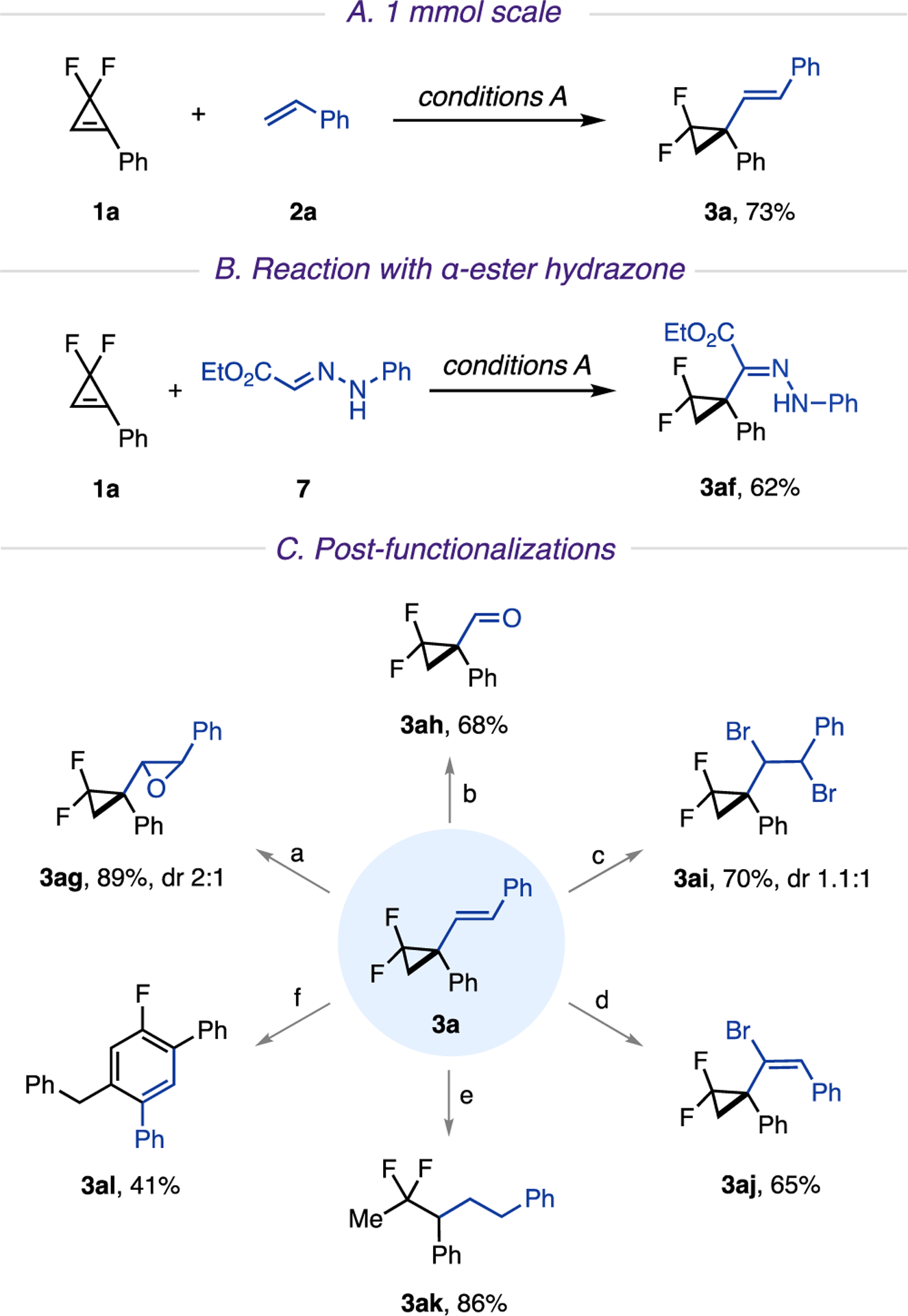
a(A) 1 mmol scale. (B) Reaction with α-ester hydrazone. (C) Postfunctionalizations. Reaction conditions: (a) m-CPBA (1.5 equiv), DCM (0.1 M), rt, 24 h; (b) 4-nitrobenzonitrile (1.5 equiv), MeCN (0.1 M), rt, 390 nm LED, 16 h; (c) LiBr (2 equiv), NaIO4 (0.5 equiv), H2SO4 (0.3 equiv), MeCN (0.05 M), rt, 24 h; (d) Br2 (1.2 equiv), DCM (0.05 M), 0 °C, 2 h, then KOH (2 equiv), THF/MeOH (1/1), 80 °C, 3 h. (e) Pd/C (10 mol %), H2 (1 atm), EtOAc (0.1 M), rt, 2 h; (f) phenylacetylene (2 equiv), Pd(TFA)2 (10 mol %), P(t-Bu)3·HBF4 (12 mol %), Cs2CO3 (2 equiv), THF (0.2 M), 60 °C, 18 h. m-CPBA: meta-chloroperoxybenzoic acid. Pd(TFA)2: palladium-(II) trifluoroacetate.
