Abstract
Background
Chronic renal failure (CKD) is associated with the presence of increased platelet reactivity and lower clinical benefit of clopidogrel. Ticagrelor has a more favorable pharmacodynamic and pharmacokinetic profile compared to clopidogrel, which has translated into better clinical outcomes in patients with acute coronary syndrome (ACS). We conducted a prospective mechanistic cohort study in order to investigate the impact of renal failure on the pharmacokinetics and pharmacodynamics of ticagrelor in patients with acute ACS.
Methods
Patients were divided into two groups based on their estimated renal clearances (eGFR ≥ 60 mL/min and eGFR < 60 mL/min). Platelet function was determined using the VerifyNow system at baseline, after the ticagrelor loading dose and at discharge. In addition, levels of ticagrelor and its active metabolite (AR-C124910XX) were determined in the first hour after loading dose.
Results
48 patients were recruited (eGFR ≥ 60 mL/min: 35 and eGFR < 60 mL/min: 13). There were no significant differences between the groups in terms of platelet inhibition after the loading or after 7 days of treatment (p = 0.219). However, the levels of ticagrelor and its active metabolite were lower in subjects with normal renal function than in CKD, especially at 4 (p = 0.02 and 0.04 respectively) and 6 h of loading (p = 0.042 and 0.08 respectively).
Conclusion
No differences in platelet inhibition were observed after treatment with ticagrelor in patients with different renal function, although patients with renal impairment showed higher levels of ticagrelor and AR-C124910XX after 4 h of the loading dose.
Keywords: Coronary artery disease, Ticagrelor, Platelet reactivity, Kidney Failure, Chronic
1. Introduction
Dual antiplatelet therapy, consisting in aspirin and a P2Y12 inhibitor, is the cornerstone of the prevention of thrombotic events in patients with coronary artery disease (CAD) [1], [2], [3]. Several clinical factors have been associated with impaired clopidogrel-induced effects. Moreover, these clinical factors are strongly related to the presence of high on-treatment platelet reactivity (HPR), which is also associated with the occurrence of adverse thrombotic events despite correct treatment compliance, including stent thrombosis. Additional common factors are diabetes mellitus, acute coronary syndrome (ACS), obesity or chronic kidney disease (CKD) [2]. Indeed, CKD is highly associated with an increased risk of atherothrombotic events in patients with CAD [4], [5]. Pharmacodynamic (PD) studies have shown that patients with impaired renal function are characterized by reduced clopidogrel-induced antiplatelet effects and higher rates of HPR compared with patients with preserved renal function [4], [6]. This observation encouraged the search for new more potent antiplatelet therapies, leading to the development of other P2Y12 receptor antagonists, such as ticagrelor.
Ticagrelor has a more favorable pharmacokinetic (PK) and PD profile than clopidogrel [7], which translated into better clinical outcomes in patients with ACS [8]. Interestingly, ticagrelor showed an impressive clinical benefit in patients with CKD in comparison with those patients without renal impairment [9]. However, PK and PD profiles of ticagrelor in ACS patients with CKD and with normal renal function, as well as rates of HPR, have been not described yet and represent the rationale for the present study.
2. Methods
2.1. Study population and design
The present is a prospective, parallel design study, aimed to compare platelet reactivity between ACS patients with normal renal function or CKD, after one week of ticagrelor treatment. We also assessed PK data in both groups. Patients recruited fulfilled the following inclusion criteria: (1) Diagnosis of non-ST elevation-ACS according to the current guidelines; (2) Patients within 12 h after the symptoms onset; (3) Received a loading dose or under chronic treatment with aspirin (100 mg/day); (4) Age between 18 and 80 years old; and (5) Body mass index (BMI) between 18 and 35 kg/m2. Exclusion criteria for this study included: known allergies to aspirin, ticagrelor or clopidogrel; concomitant use of oral anticoagulants; hemoglobin < 10 mg/dL; platelet count < 80x106/mL, blood dyscrasias; active bleeding or hemodynamic instability; patients on hemodialysis or peritoneal dialysis; a change in estimated glomerular filtration rate (eGFR) ≥ 15 mL/min/1.73 m2 within 90 days prior to enrollment or eGFR < 15 mL/min/1.73 m2, known infectious diseases or neoplasia, baseline ALT > 2.5 times the upper limit of normal, sick sinus syndrome or high degree AV block without pacemaker protection, drugs interfering CYP3A4 metabolism (to avoid interaction with ticagrelor, i.e. ketoconazole, itraconazole, clarithromycin, ritonavir, etc.), and pregnant females. All candidates were screened in the Cardiology Department of Hospital Clínico Universitario Virgen de la Arrixaca (Murcia, Spain). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
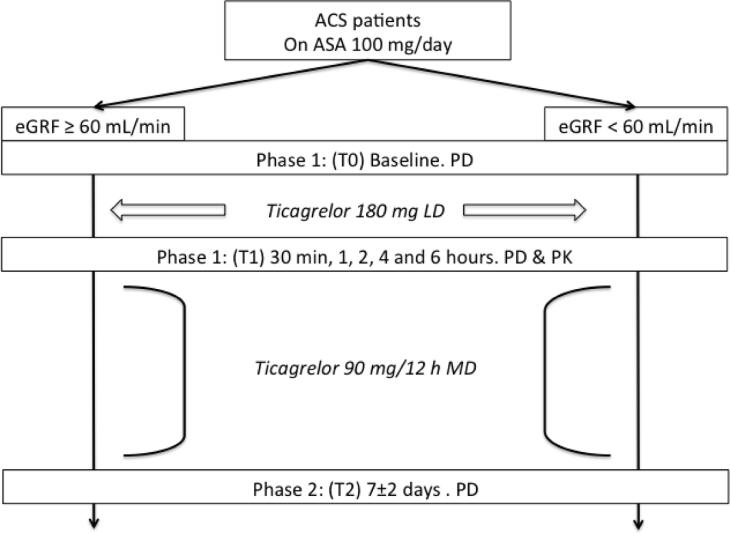
An informed consent was obtained from each patient. They were divided according to their renal function as normal (eGFR ≥ 60 mL/min/1.73 m2) and CKD (eGFR < 60 mL/min/1.73 m2) assessed by the Cockcroft-Gault formula [10]. Patients were treated with a loading dose (LD) of 180 mg of ticagrelor at the enrolling and maintenance ticagrelor regiment (90 mg/twice daily) for at least 7 ± 2 days. After this period, the referring attending physician decided to maintain ticagrelor or switch to another antiplatelet agent. Investigators and patients were aware of treatment assignment. However, laboratory personnel were blinded to treatment assignment. Compliance to aspirin and ticagrelor was assessed by interview and pill counting. Blood sampling for the study was divided in two phases: Phase 1 included baseline during treatment with aspirin (T0) and blood samples at 30 min, 1, 2, 4 and 6 h after 180 mg loading dose of ticagrelor (T1); Phase 2 involved 7 ± 2 days of maintenance treatment of ticagrelor 90 mg twice a day (T2). A flow diagram of the study is presented (Fig. 1).
Fig. 1.
Study design.
2.2. Blood sampling and laboratory assessments
Peripheral venous blood samples were drawn through a short venous catheter inserted into a forearm vein and collected in citrate and serum tubes at each study time point for all laboratory assessments. The first 2–4 mL of blood were discarded to avoid spontaneous platelet activation.
Platelet function testing was carried out by VerifyNow System, a turbidimetric based optical detection system which measures platelet induced aggregation as an increase in light transmittance (Accumetrics, San Diego, CA) and was utilized according to manufacturer’s instructions, as previously described [11], [12]. On the other hand, ticagrelor active metabolite assessment was performed with liquid chromatography with tandem mass spectrometry. This assay has been performed at phase 1, in timepoints (T1) including baseline and at 30 min, 1, 2, 4 and 6 h after ticagrelor LD. Venous blood samples (2 mL) were collected into lithium heparin tubes. Plasma samples were prepared by centrifugation (1500 g, 10 min, 4 °C) within 30 min, and stored frozen (-20 °C) until analyzed. Ticagrelor and AR-C124910XX plasma concentrations were analyzed, after protein precipitation, using a fully validated liquid chromatography with the tandem mass spectrometry method [13], [14]. Lower limits of quantification were 5 ng/ml (ticagrelor) and 2.5 ng/ml (AR-C124910XX). Covance Laboratories Inc. – Indianapolis, Indiana (USA) performed these experiments.
In addition, hematological and biochemical parameters were obtained, including routine hemogram, lipid profile, glucose, and creatinine clearance.
2.3. Statistical analysis
The primary endpoint was the comparison of the PRU values determined by VerfifyNow-P2Y12 system between normal renal function and CKD patients after 7 ± 2 days of treatment with ticagrelor. Given the lack of preliminary data in this field, we chose an arbitrary sample size of 60 patients (30 patients in each group) according to previous investigations [15], [16] and in line with recommendations for pilot investigations [17]. However, we had to finish the recruitment prematurely because of the low inclusion rate mainly due to concomitant anticoagulant therapy and the initial use of ticagrelor.
Continuous variables were expressed as a mean ± standard deviation (SD) or median [interquartile range, IQR], when appropriate. Categorical variables were expressed as frequencies and percentages (%). Paired Student’s T test or Wilcoxon T test was used to compare continuous variables. Comparisons between categorical variables were performed using McNemar test or binomial exact test. Per protocol, missing data were not imputated. Differences between groups depending on renal function were studied by the unpaired T test for independent samples, or the Mann-Whitney U test (as appropriate) for continuous variables. Influence of the antiplatelet therapy or the renal function at different time points of platelets function tests or biomarkers levels were performed by analysis of variance for repetitive measures for one between-subjects factor. Both factors can be studied simultaneously by analysis of variance (ANOVA) for repetitive measures for two between-subjects factors. To control the influence of other covariables affecting or interfering measures (as different distribution of patients with diabetes mellitus between groups), analysis of covariance (ANCOVA) has been carried out. A two-side p-value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.15.0 software (SPSS Inc. Chicago, IL).
3. Results
3.1. Recruitment and baseline characteristics
Between December 2015 and February 2019, 200 patients were screened in the Emergency Department after being accepted for admission in the Cardiology Department. Of them, 52 patients meeting study inclusion criteria were identified and provided their written consent to participate in the study; and 2 patients withdrew after screening. Thereafter, 1 patient was excluded since underwent hemodialysis therapy during the hospital stay; and 1 patient was excluded because underwent urgent percutaneous coronary intervention, thus missing several time points, giving a final sample size of 48 patients (CKD, n = 13; preserved renal function, n = 35).
Baseline characteristics are depicted in Table 1. In brief, CKD patients were older with a numerical tendency to present with more previous atherosclerotic disease (more previous myocardial infarction and cerebrovascular disease). Also, there was a numerical tendency to higher percentage of cardiovascular risk factors among CKD patients.
Table 1.
Basal characteristics,
| Total | CKD | Normal | P value | |
|---|---|---|---|---|
| N | 48 | 13 | 35 | |
| Age | 65.9 ± 10.9 | 75.1 ± 6.3 | 62.5 ± 10.3 | <0.001 |
| Gender male | 36 (75%) | 9 (69.2%) | 27 (77.1%) | 0.574 |
| BMI | 28.1 ± 4.3 | 28.8 ± 3.8 | 27.9 ± 4.5 | 0.636 |
| Hypertension | 37 (77.1%) | 12 (92.3%) | 25 (71.4%) | 0.126 |
| DM | 23 (47.9%) | 6 (46.2%) | 17 (48.6%) | 0.826 |
| Dyslipemia | 33 (68.8%) | 10 (76.9%) | 23 (65.7%) | 0.457 |
| Smoker | 15 (31.3%) | 5 (38.5%) | 21 (60.0%) | 0.100 |
| PAD | 4 (8.3%) | 0 | 4 (11.4%) | 0.203 |
| Previous ACV/TIA | 5 (10.4%) | 3 (23.1%) | 2 (5.7%) | 0.080 |
| Previous MI | 18 (37.5%) | 7 (53.8%) | 11 (31.4%) | 0.154 |
| Creatinine (mg/dL) | 1.0 ± 0.38 | 1.38 ± 0.50 | 0.87 ± 0.21 | 0.003 |
| eGFR (mL/min) | 137.4 ± 365.3 | 46.7 ± 8.8 | 97.4 ± 32.6 | < 0.001 |
BMI: Body mass index; DM: Diabetes mellitus, PAD: Peripheral artery disease, ACV: Acute cerebrovascular disease; TIA: Transitory isquemic attack; MI: Myocardial infarction. eGFR: estimated glomerular filtration rate. CKD: Chronic kidney disease.
All patients were admitted for moderate-high risk ACS, with an estimated GRACE risk score of 127.7 ± 36.3 (CKD: 137.5 ± 51.3 vs. Normal: 123.4 ± 30.3; p = 0.268). More than half of these patients presented with multivessel coronary disease. There were no differences in presentation between both groups (Table 2).
Table 2.
Index event characteristics.
| Total | CKD | Normal | P value | |
|---|---|---|---|---|
| ECG: | ||||
| Normal | 12 (25.5%) | 5 (38.5%) | 7 (20.6%) | 0.616 |
| Descent ST | 20 (42.6%) | 4 (30.8%) | 16 (47.1%) | |
| T negative | 8 (17.0%) | 2 (15.4%) | 6 (17.6%) | |
| Indetermined | 7 (14.9%) | 2 (15.4%) | 5 (14.7%) | |
| Hs TnT peak | 68.0 (21.5–116.0) | 94.0 (25.5–346.6) | 66.0 (18.0–114.0) | 0.188 |
| LVEF (%) | 54.2 ± 11.2 | 48.6 ± 16.7 | 56.2 ± 7.7 | 0.134 |
| Coronary anatomy | ||||
| LM | 1 (2.1%) | 0 | 1 (2.9%) | 0.532 |
| 0 | 7 (14.9%) | 1 (7.7%) | 6 (17.6%) | 0.637 |
| 1 | 14 (29.8%) | 3 (23.1%) | 11 (32.4%) | |
| 2 | 16 (34.0%) | 16 (46.2%) | 10 (29.4%) | |
| 3 | 10 (21.3%) | 3 (23.1%) | 7 (20.6% | |
| Grace risk score | 127.7 ± 36.3 | 137.5 ± 51.3 | 123.4 ± 30.3 | 0.268 |
Hs TnT: High sensitivity Troponin T. LVEF: Left ventricle ejection fraction. LM: Left main. CKD: Chronic kidney disease.
3.2. Platelet function
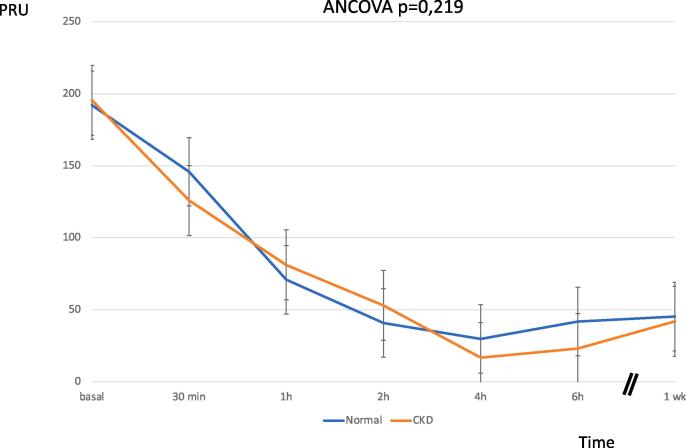
After ticagrelor 180 mg LD, platelet reactivity decreased significantly, reaching about 90% of reduction at 4 h after (CKD: PRU baseline 192.0 ± 93.8vs. PRU 4 h 29.7 ± 30.1; Normal: PRU Baseline 195.5 ± 68.3vs. PRU 4 h 16.7 ± 27.7). There was no difference between groups regarding to the platelet inhibition (p = 0.219). In addition, there was observed a strong platelet inhibition by PRU at 1 week, without differences between groups (CKD 45.2 ± 63.2 vs. Normal: 41.9 ± 50.4, p = 0.856) (Fig. 2).
Fig. 2.
Platelet function profiles of normal and CKD patients over the time, assessed by VerifyNow-P2Y12 system. ACS: acute coronary syndrome, ASA: aspirin. eGRF: estimated glomerular filtration. PD: pharmacodynamics, PK: pharmacokinetics, PRU: Platelet Reactivity Units.
3.3. Pharmacokinetic assessment
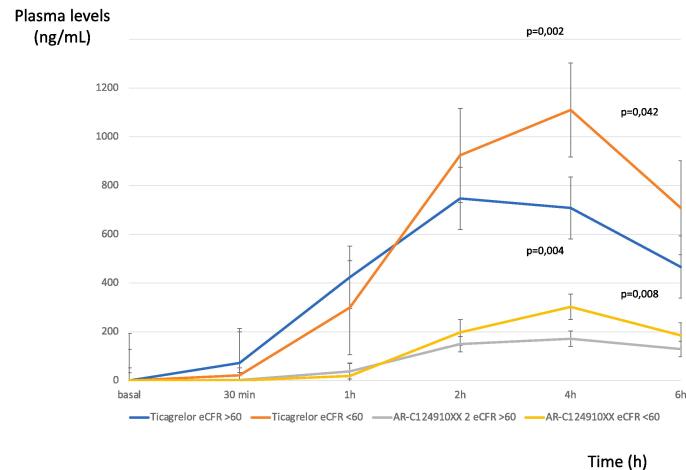
After ticagrelor 180 mg LD, ticagrelor levels were lower in patients with normal renal function compared to CKD patients, particularly at 4 h [CKD:1110.0 (924.0–1370.0) ng/mL vs. Normal:708.0 (538.5–940.0) ng/mL; p = 0.002] and at 6 h [CKD:708.5 (456.3–1055.3) ng/mL vs. Normal:466.0 (350.8682.3) ng/mL; p = 0.042] after LD. Also, AR-C124910XX showed lower levels in normal patients compared with CKD patients, mainly at 4 and 6 h after ticagrelor LD [at 4 h CKD: 302.0 (225.0–312.0) ng/mL vs. normal: 171.5 (132.5–247.5) ng/mL; p = 0.004; at 6 h CKD: 185.5 (161.8–204.5) ng/mLvs. Normal: 129.0 (103.3–172.8) ng/mL; p = 0.008] (Fig. 3).
Fig. 3.
AR-C124910XX and ticagrelor plasma levels [ng/mL] over the time in normal and CKD patients.
4. Discussion
4.1. PK and PD profile of ticagrelor
Initial data in ticagrelor PK and PD profiles have come from healthy volunteers [18]. Recent investigation conducted in CKD patients has demonstrated an improved PD and PK profile of ticagrelor in comparison with clopidogrel in ACS setting [19]. However, a direct comparison of PK and PD profiles of ticagrelor in ACS according to renal function has not been performed yet. The present study was designed trying to address this question. After ticagrelor LD, there was a strong and consistent decrease in platelet reactivity reaching the peak at 4 h, which is in line with previous studies including acute patients (with ACS) [20]. Moreover, there was no difference between both CKD and normal groups in terms of platelet inhibition. The inhibition remains at 1 week, without differences between groups. Thus, the strong inhibition among CKD patients (and similar to normal patients) may explain the maintained clinical benefit among both groups showed in the clinical trial [9].
On the other hand, there were higher levels of ticagrelor and its active metabolite (AR-C124910XX) in CKD patients in comparison with normal renal function patients, showing a peak at 4 h. Although these differences in both ticagrelor and AR-C124910XX levels did not confer different levels in platelet inhibition, this “improved” PK profile may be one of the plausible reasons explaining the similar platelet inhibition levels showed in normal renal function and CKD patients. Moreover, these excess of ticagrelor and AR-C124910XX levels may act through the inhibition of adenosine reuptake. This effect has been related to beneficial outcomes (such as improvement in myocardial perfusion), which could be more important in patients with impaired renal function [21], [22]. Thus, we could hypothesize that these may be some reasons for the impressive clinical benefit in patients with CKD in comparison with patients without renal impairment found in PLATO trial [9]. However, this observation is matter of debate [6] and remains poor understood. The ongoing TicagRelorOr Clopidogrel in severe or terminal chronic kidney patients Undergoing PERcutaneous coronary intervention for acute coronary syndrome (TROUPER) trial [23], has been designed to compare the efficacy of ticagrelor and clopidogrel in stage > 3b CKD patients presenting with ACS and scheduled for an invasive strategy.
The question is why ticagrelor showed a different PK profile in CKD patients when no effect of renal function has been reported previously [9], [24]. It is well known that CKD associates a higher rate of intoxication by certain drugs, even when they do not require dose adjustment due to renal function, since CKD can affect different PK stages [25], [26]. A number of studies indicate that intestinal function could be altered in CKD, which would also affect the bioavailability of drugs. These changes may be related to a decrease in the first pass metabolism or a reduction in intestinal extrusion by membrane transporters. In fact, some authors have reported the inhibition of some isoforms of cytochrome P450 located in the enterocyte, thus inhibiting this first pass intestinal metabolism [27]. Other authors have related a decrease in intestinal expression of membrane transporters, such as P-glycoprotein and multidrug-resistance-related protein (MRP) 2, as contributor in the increased bioavailability of drugs in CKD [28].
Compounds as tacrolimus exhibits hepatic and intestinal metabolism after oral administration, is also influenced by renal function with a decrease in its bioavailability in animal models. Specifically, the drug concentration is increased by 35% in uremic rats compared to normal controls [29]. This is interesting since ticagrelor is also a substrate of the CYP3A4 isoform of cytochrome P450, and this enzyme is responsible of the formation of the major active metabolite of ticagrelor (AR-C124910XX) [7]. Furthermore, ticagrelor has a molecular weight of 522,567 g/mol, which is interesting if we consider that Kimura et al. reported a higher intestinal absorption of molecules with molecular weight < 1000 in another experimental model of renal failure [30].
These phenomena may explain the higher ticagrelor and AR-C124910XX levels in CKD patients observed in the present study. However, this is the first study showing such difference in ACS patients, in whom the proved pleiotropic effect of ticagrelor, different from the one mediated by P2Y12 inhibition, may explain clinically relevant benefits. For example, many studies reported that ticagrelor increases adenosine plasma levels in patients with ACS [31]. More recently, it has been demonstrated an influence in the metabolic pathways of amino acids (cysteine, methionine, phenylalanine, tyrosine and tryptophan) and phospholipids (glycerophosphoethanolamines and glycerophosphoserines) in the stable phase [32], [33].
The present study may explain the clinical benefit showed by ticagrelor in patients with CKD, encouraging further investigation in this topic. The hypothesis of better antiplatelet responses due to an improved pharmacokinetic profile in renal failure merits more focused research, since it could reinforce the use of this agent in this clinical setting. Additionally, it is important to highlight that CKD is associated with other vascular territories disease, thus our results are potentially translatable to a broad range of CKD patients who may require antiplatelet therapy (i.e., cerebrovascular, pheripheral disease).
4.2. Study limitations
There are some limitations to acknowledge. The first one is the small size, particularly in the CKD group. CKD patients are underrepresented in the majority of studies due to the difficulties to fulfill all inclusion and exclusion criteria. Second, there is no receptor binding analysis. Thus, the relationship between ticagrelor levels and PD effect is not directly tested. Third, the grouping did not follow the principle of a single variable (i.e., there was a significant difference between the CKD group and the normal group). Also, we did not genotype for cytochrome polymorphisms (mainly CYP3A4) which may be related in the differences reported [34]. In addition, a small part of ticagrelor is metabolized by CYP3A5, so we cannot exclude the influence of different genotypes in the research conclusion. Other limitation to acknowledge is that the known pleiotropic effect attributed to ticagrelor was not assessed in the present study. Considering the increase of ticagrelor’s metabolite in this population, these pleiotropic effects might be enhanced among CKD patients, representing another cause to explain the improved clinical profile. Finally, although we propose some possible explanations, this study is not designed to clarify the mechanism why patient with renal impairment showed higher levels of ticagrelor and AR-C124910XX, thus further investigation is needed.
5. Conclusion
Ticagrelor loading dose achieves similar platelet inhibition in CKD and normal renal function patients admitted for non-ST elevation ACS. However, there is a significant increase in ticagrelor and AR-C124910XX levels in CKD patients compared with those with normal renal function.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was conducted with support from AstraZeneca Farmacéutica Spain, S.A.
References
- 1.Collet J.P., Thiele H., Barbato E., et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 2.Ferreiro J.L., Angiolillo D.J. Clopidogrel response variability: current status and future directions. ThrombHaemost. 2009;102:7–14. doi: 10.1160/TH09-03-0185. [DOI] [PubMed] [Google Scholar]
- 3.Sofi F., Marcucci R., Gori A.M., Giusti B., Abbate R., Gensini G.F. Clopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated meta-analysis. ThrombHaemost. 2010;103:841–848. doi: 10.1160/TH09-06-0418. [DOI] [PubMed] [Google Scholar]
- 4.Capodanno D., Angiolillo D.J. Antithrombotic therapy in patients with chronic kidney disease. Circulation. 2012;125:2649–2661. doi: 10.1161/CIRCULATIONAHA.111.084996. [DOI] [PubMed] [Google Scholar]
- 5.Bonello L., Angiolillo D.J., Aradi D., Sibbing D. P2Y12-ADP Receptor Blockade in Chronic Kidney Disease Patients With Acute Coronary Syndromes. Circulation. 2018;138:1582–1596. doi: 10.1161/CIRCULATIONAHA.118.032078. [DOI] [PubMed] [Google Scholar]
- 6.Montalescot G., Silvain J. Ticagrelor in the renal dysfunction subgroup: subjugated or substantiated? Circulation. 2010;122:1049–1052. doi: 10.1161/CIRCULATIONAHA.110.974683. [DOI] [PubMed] [Google Scholar]
- 7.FDA ticagrelor label package: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM264004.pdf.
- 8.Wallentin L., Becker R.C., Budaj A,: PLATO Investigators., et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 9.James S., Budaj A., Aylward P., et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122:1056–1067. doi: 10.1161/CIRCULATIONAHA.109.933796. [DOI] [PubMed] [Google Scholar]
- 10.Cockcroft D. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Storey R.F., Angiolillo D.J., Patil S.B., et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J. Am. Coll. Cardiol. 2010;56:1456–1462. doi: 10.1016/j.jacc.2010.03.100. [DOI] [PubMed] [Google Scholar]
- 12.Sibbing D., Braun S., Jawansky S., et al. Assessment of ADP-induced platelet aggregation with light transmission aggregometry and multiple electrode platelet aggregometry before and after clopidogrel treatment. Thromb. Haemost. 2008;99:121–126. doi: 10.1160/TH07-07-0478. [DOI] [PubMed] [Google Scholar]
- 13.Teng R., Maya J., Butler K. Evaluation of the pharmacokineticsandpharmacodynamics of ticagrelor co-administered with aspirin in healthyvolunteers. Platelets. 2013;24:615–624. doi: 10.3109/09537104.2012.748185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sillén H., Cook M., Davis P. Determination of ticagrelor and twometabolites in plasma samples by liquidchromatography and mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010;878:2299–2306. doi: 10.1016/j.jchromb.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Angiolillo D.J., Bernardo E., Capodanno D., et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J. Am. Coll. Cardiol. 2010;55:1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 16.Tello-Montoliu A., Ferreiro J.L., Kodali M.K., et al. Impact of renal function on clopidogrel-induced antiplatelet effects in coronary artery disease patients without diabetes mellitus. J. Thromb. Thrombolysis. 2013;36:14–17. doi: 10.1007/s11239-012-0828-1. [DOI] [PubMed] [Google Scholar]
- 17.Lancaster G.A., Dodd S., Williamson P.R. Design and analysis of pilot studies: recommendations for good practice. J. Eval. Clin. Pract. 2004;10:307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 18.Butler K., Teng R. Pharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with severe renal impairment. J. Clin. Pharmacol. 2012;52:1388–1398. doi: 10.1177/0091270011415526. [DOI] [PubMed] [Google Scholar]
- 19.Wang H., Qi J., Li Y., Tang Y., Li C., Li J., Han Y. Pharmacodynamics and pharmacokinetics of ticagrelor vs. clopidogrel in patients with acute coronary syndromes and chronic kidney disease. Br. J. Clin. Pharmacol. 2018;84:88–96. doi: 10.1111/bcp.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexopoulos D., Xanthopoulou I., Gkizas V., et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2012;5:797–804. doi: 10.1161/CIRCINTERVENTIONS.112.972323. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong D., Summers C., Ewart L., et al. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J. Cardiovasc. Pharmacol. Ther. 2014;19:209–219. doi: 10.1177/1074248413511693. [DOI] [PubMed] [Google Scholar]
- 22.Aungraheeta R., Conibear A., Butler M., et al. Inverse agonism at the P2Y12 receptor and ENT1 transporter blockade contribute to platelet inhibition by ticagrelor. Blood. 2016;128:2717–2728. doi: 10.1182/blood-2016-03-707844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laine M., Lemesle G., Burtey S., Cayla G., Range G., Quaino G., Canault M., Pankert M., Paganelli F., Puymirat E., Bonello L. TicagRelorOr Clopidogrel in severe or terminal chronic kidney patients Undergoing PERcutaneous coronary intervention for acute coronary syndrome: The TROUPER trial. Am. Heart J. 2020;225:19–26. doi: 10.1016/j.ahj.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Husted S., van Giezen J.J. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc. Ther. 2009;27:259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolin T.D. Altered nonrenal drug clearance in ESRD. Curr. Opin. Nephrol. Hypertens. 2008;17:555–559. doi: 10.1097/MNH.0b013e3283136732. [DOI] [PubMed] [Google Scholar]
- 26.Naud J., Nolin T.D., Leblond F.A., Pichette V. Current understanding of drug disposition in kidney disease. J. Clin. Pharmacol. 2012;52:10–22. doi: 10.1177/0091270011413588. [DOI] [PubMed] [Google Scholar]
- 27.Leblond F.A., Petrucci M., Dube P., et al. Downregulation ofintestinal cytochrome p450 in chronic renal failure. J. Am. Soc. Nephrol. 2002;13:1579–1585. doi: 10.1097/01.asn.0000017575.50319.77. [DOI] [PubMed] [Google Scholar]
- 28.Naud J., Michaud J., Boisvert C., Desbiens K., Leblond F.A., Mitchell A., Jones C., Bonnardeaux A., Pichette V. Down-regulation of intestinal drug transporters in chronic renal failure in rats. J. Pharmacol. Exp. Ther. 2007;320:978–985. doi: 10.1124/jpet.106.112631. [DOI] [PubMed] [Google Scholar]
- 29.Okabe H., Hashimoto Y., Inui K.I. Pharmacokinetics and bioavailabilityof tacrolimus in rats with experimental renal dysfunction. J. Pharm. Pharmacol. 2000;52:1467–1472. doi: 10.1211/0022357001777676. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T., Kobayashi A., Kobayashi M., Numata K., Kawai Y., Nakayama T., Mori M., Awai M. Intestinal absorption of drugs in rats with glycerol-inducedacute renal failure. Chem. Pharm. Bull. 1988;36:1847–1856. doi: 10.1248/cpb.36.1847. [DOI] [PubMed] [Google Scholar]
- 31.Bonello L., Laine M., Kipson N., Mancini J., Helal O., Fromonot J., Gariboldi V., Condo J., Thuny F., Frere C., Camoin-Jau L., Paganelli F., Dignat-George F., Guieu R. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J. Am. Coll. Cardiol. 2014;63:872–877. doi: 10.1016/j.jacc.2013.09.067. [DOI] [PubMed] [Google Scholar]
- 32.Tam C.F., Chan Y.H., Wong Y.K., Li Z., Zhu X., Su K.J., Ganguly A., Hwa K., Ling X.B., Tse H.F. Multi-Omics Signatures Link to Ticagrelor Effects on Vascular Function in Patients With Acute Coronary Syndrome. Arterioscler. Thromb. Vasc. Biol. 2022;42:789–798. doi: 10.1161/ATVBAHA.121.317513. [DOI] [PubMed] [Google Scholar]
- 33.Pavasini R., Campo G. Ticagrelor and Endothelial Function: An Effect That Persists Far From the Acute Phase and in Monotherapy. Arterioscler. Thromb. Vasc. Biol. 2022;42:799–801. doi: 10.1161/ATVBAHA.122.317693. [DOI] [PubMed] [Google Scholar]
- 34.Liu S., Shi X., Tian X., Zhang X., Sun Z., Miao L. Effect of CYP3A4∗1G and CYP3A5∗3 Polymorphisms on Pharmacokinetics and Pharmacodynamics of Ticagrelor in Healthy Chinese Subjects. Front. Pharmacol. 2017;8:176. doi: 10.3389/fphar.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]