Abstract
The apoptotic caspase subfamily evolved into two subfamilies—monomeric initiators and dimeric effectors; both subfamilies share a conserved caspase–hemoglobinase fold with a protease domain containing a large subunit and a small subunit. Sequence variations in the conserved caspase–hemoglobinase fold resulted in changes in oligomerization, enzyme specificity, and regulation, making caspases an excellent model for examining the mechanisms of molecular evolution in fine-tuning structure, function, and allosteric regulation. We examined the urea-induced equilibrium folding/unfolding of two initiator caspases, monomeric caspase-8 and cFLIPL, over a broad pH range. Both proteins unfold by a three-state equilibrium mechanism that includes a partially folded intermediate. In addition, both proteins undergo a conserved pH-dependent conformational change that is controlled by an evolutionarily conserved mechanism. We show that the conformational free energy landscape of the caspase monomer is conserved in the monomeric and dimeric subfamilies. Molecular dynamics simulations in the presence or the absence of urea, coupled with limited trypsin proteolysis and mass spectrometry, show that the small subunit is unstable in the protomer and unfolds prior to the large subunit. In addition, the unfolding of helix 2 in the large subunit results in disruption of a conserved allosteric site. Because the small subunit forms the interface for dimerization, our results highlight an important driving force for the evolution of the dimeric caspase subfamily through stabilizing the small subunit.
Keywords: caspase, apoptosis, folding landscape, protein evolution, evolutionary biology, protease, protein folding, fluorescence emission, CD, molecular dynamics simulations
Caspases are a family of enzymes that play critical roles in apoptosis and inflammation. In the apoptotic cascade, caspases function either in the intrinsic pathway or in the extrinsic pathway, depending on the origin of the signal for apoptosis (1). In the extrinsic pathway of apoptosis, caspases evolved into two distinct subfamilies, namely initiator caspases and effector caspases, and their activation mechanisms differ yet are critical for the regulation of apoptosis (2). Caspases-8 and -10 are initiators of apoptosis, whereas caspases-3, -6, and -7 are effectors of apoptosis (3). Caspases are expressed in cells as zymogens and must be activated for full enzyme activity. The initiator procaspases exist as monomers and must dimerize to gain partial activity; dimerization is followed by cleavage of the zymogen, leading to full catalytic potential. In contrast, the effector procaspases-3, -6, and -7 are stable dimers and require only proteolytic processing to be activated (4, 5).
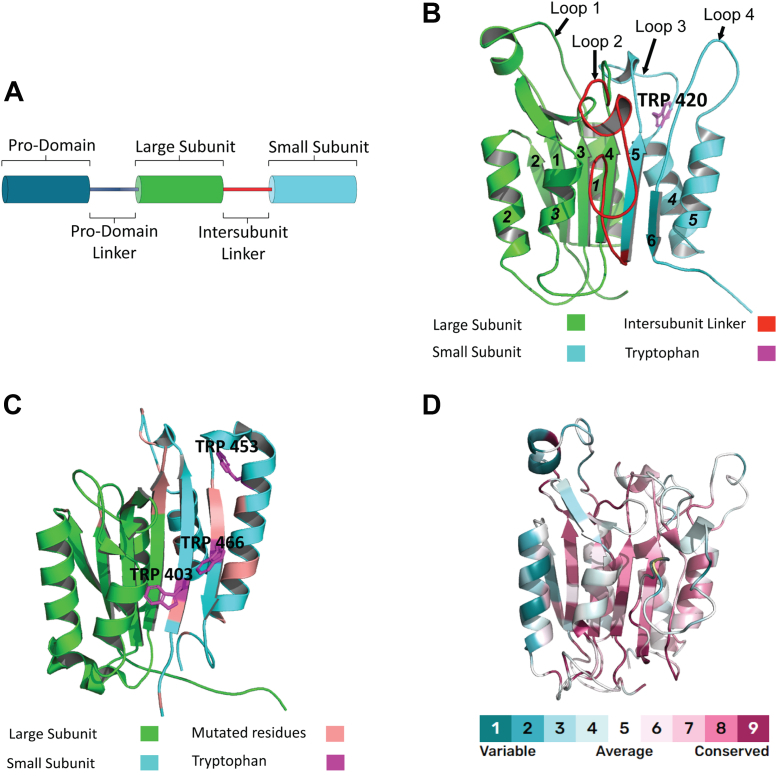
Caspases are an attractive system to study protein evolution because of the evolutionarily conserved fold that is utilized in both monomeric and dimeric subfamilies (6). The caspase–hemoglobinase fold that comprises the protease domain has a Rossmann-like organization (three-layer sandwich) and has been largely conserved for at least one billion years of evolution, although the sequence conservation is generally low (∼15%) (7, 8). The caspase protomer is organized as a single polypeptide chain with an N-terminal prodomain connected to the protease domain, and the protease domain is organized with a large subunit, intersubunit linker (IL), and small subunit (Fig. 1A) (5). Although the zymogens of caspases-8 and -10 are produced as monomers in the cell, the proteins are enzymatically active only in the dimeric state (4). The death effector domains (DEDs) or caspase activation and recruitment domains within the prodomain of initiator caspases facilitate dimerization through interactions with similar motifs on oligomerization platforms, such as the death-inducing signaling complex (9). The IL is cleaved following dimerization on the death-inducing signaling complex or other platforms, which separates the large and small subunits and allows the active site to form. The two subunits of the protomer fold into a single unit with a six-stranded β-sheet core and five α-helices on the surface (Fig. 1B). Cleavage of the IL (loop 2 in Fig. 1B) leads to active-site loop rearrangements and formation of the substrate-binding pocket (10).
Figure 1.
Caspase structure and conservation.A, domain organization of initiator caspases. B, structure of caspase-8 (Protein Data Bank ID: 6PX9) showing sequence of beta sheets (bold numbers) and helices (bold italics) in the protease domain. Large subunit, intersubunit linker, and small subunit are color coded in accordance with the legend. In addition, the only tryptophan in caspase 8 is labeled in magenta. C, structure of CflipL (Protein Data Bank ID: 3H11) showing tryptophan residues in magenta. Residues that are conserved in all caspases but mutated in CflipL are shown in salmon. Large subunit and small subunit are color coded as per the legend, whereas the intersubunit linker is missing as the crystal structure is that of processed mature enzyme. D, amino acid conservation of chordate caspases-3, -6, -7, -8, -10, and Cflipl is illustrated on the structure of caspase-8 using the ConSurf conservation coloring scheme on the structure of procaspase-8 (Protein Data Bank ID: 6PX9).
In addition to its role in apoptosis, caspase-8 performs a range of nonapoptotic functions. For example, it forms a heterodimer with cFLIPL, a pseudoenzyme, and the caspase-8–cFLIPL heterodimer functions in prosurvival pathways by blocking RIP1 and RIP3 from initiating necroptosis, a nonapoptotic type of cell death (11). The cFLIPL–cFLAR gene is located in close proximity to those of caspases-8 and -10 on human chromosome 2q33 to 34, and the protein is structurally similar to caspase-8 and -10 but lacks a functional protease domain (12). cFLIPL evolved early in the caspase-8/-10 subfamily, and while it retains the caspase–hemoglobinase fold, mutations in the active site prevent substrate binding (Figs. 1C and S1) (13).
A comparison of the amino acid sequences of extrinsic caspases from all chordates shows that the β-sheet core is highly conserved, except for β-strand 2, but the helices on the protein surface are less conserved, particularly helix 2 (Fig. 1D). Overall, the conservation of the caspase structural scaffold makes it an excellent model for understanding the evolutionary events that led to species-specific changes in oligomerization, enzyme specificity, and regulation. Indeed, it is not clear how the conserved fold resulted in both monomeric and dimeric subfamilies or how oligomerization evolved as a key regulatory mechanism for caspase activity.
The assembly of the effector caspase dimer has been studied extensively, but little is known about the conformational landscape of the initiator caspases (6, 14). Studies of effector caspases-3, -6, and -7 from humans as well as caspase-3 from zebrafish show that the dimers fold and unfold via a four-state equilibrium pathway in which both dimeric and monomeric partially folded intermediates are well populated (6, 14, 15). For the effector caspases, the folding was found to follow a four-state pathway (N2↔I2↔2I↔2U) in which the native dimer (N2) unfolds to a partially folded dimeric intermediate (I2), which dissociates into a partially folded monomer (I) prior to unfolding (U). While the folding landscape is conserved, differences in the relative population of the folding intermediates provide flexibility for each caspase. Furthermore, studies of the common ancestor of effector caspases showed that the folding landscape was established more than 650 million years ago (6, 16). Overall, dimerization is important to the overall conformational free energy in a conserved folding landscape through contributing an additional 14 to 18 kcal mol−1 of free energy to the native dimer compared with that of the monomeric folding intermediate (5–7 kcal mol−1) (6, 14).
Aside from the conformational free energy obtained for the monomeric folding intermediate of dimeric caspases, there are no data on the folding of the monomeric caspases. That is, to date, the folding landscape of the protomer has been studied only in the context of a folding intermediate during dimer formation. Here, we examined the equilibrium folding and unfolding of human caspase-8 and cFLIPL, and we show that the protomer folds through at least one well-populated partially folded intermediate prior to forming the native protein (N↔I↔U). The native protein is most stable near physiological pH and exhibits substantial loss of secondary structure at both lower and higher pH, such that the partially folded intermediate, I, predominates. In addition, data from molecular dynamics (MD) simulations at several pHs and in the presence of urea (5 M), followed by limited proteolysis and mass spectrometry, show that the small subunit is unstable within the protomer and unfolds prior to the large subunit. Finally, we showed previously that effector caspases undergo a pH-dependent conformational change, with a pKa of ∼6 and that the conformational change resulted in an inactive enzyme, although the protein remained in the dimeric state (6, 15, 17). We observe a similar pH-dependent conformational change in the caspase-8 and cFLIPL protomers, suggesting that the effects of pH on the protein conformation may be due to an evolutionarily conserved mechanism. Altogether, the data show a conserved folding landscape for caspases and a role for dimerization in stabilizing the small subunit within the protomer as well as an evolutionarily conserved pH-dependent conformational change present in all caspases.
Results
Caspase-8 and cFLIPL have intrinsic fluorescence probes that can be used to monitor conformational changes
The construct of caspase-8 that we utilized comprises 264 amino acids (30 kDa), whereas that of cFLIPL is 294 amino acids (33.9 kDa) (Fig. S1) (18). Both constructs lack the prodomain. Caspase-8 has one tryptophan residue (W420) in active-site loop L3, and the tryptophan lines the S2 and S4 substrate-binding pockets in the active structure (Figs. S1 and 1B). In contrast, cFLIPL contains three tryptophans (Figs. S1 and 1C): W403 on β-sheet 5, W466 on β-sheet 6, and W453 on α-helix 5. Thus, for both proteins, the tryptophans reside in the small subunit, and in cFLIPL, one tryptophan (W453) forms part of the dimerization interface. In addition, caspase-8 and cFLIPL have 12 tyrosines (Fig. S1) that are well distributed throughout the structures. As described previously for effector caspases (6, 14, 15), we examined conformational changes in caspase-8 and cFLIPL in the presence and absence of urea by observing changes in fluorescence emission following excitation at 280 nm or at 295 nm. While excitation at 280 nm monitors fluorescence emission of tryptophan and tyrosine residues, excitation at 295 nm is specific for tryptophan residues (19). We also monitored changes in secondary structure during unfolding via far-UV CD.
The folding of caspase-8 and cFLIPL includes multiple intermediates
Native caspase-8 (that is, in the absence of urea) exhibits fluorescence emission maxima at 320 nm (Fig. S2A) or 338 nm (Fig. S2B) when excited at 280 nm or 295 nm, respectively, and far-UV CD spectra consistent with a well-packed secondary structure (Fig. S2C). In comparison, native cFLIPL exhibits fluorescence emission maxima at 340 nm when excited at 280 nm (Fig. S2D) or 295 nm (Fig. S2E), suggesting that one or more tryptophan residues in cFLIPL is more exposed to solvent than is the single tryptophan in caspase-8. Like caspase-8, cFLIPL also exhibits far-UV spectra consistent with well-packed secondary structure (Fig. S2F). When caspase-8 (Fig. S2, A and B) and cFLIPL (Fig. S2, D and E) are incubated in 9 M urea-containing buffer at pH 7.5, both proteins exhibit a red shift in fluorescence emission to 350 nm as well as a loss of secondary structure, demonstrating that the tryptophans are exposed to solvent and that the proteins are largely unfolded (Fig. S2). In addition, at intermediate (4 M) to maximum (9 M) urea concentrations, caspase-8 exhibits two peaks in the fluorescence emission profile when excited at 280 nm, where emission maxima are observed at 305 and 355 nm (Fig. S2A). As described previously for an ancestral caspase, the two peaks likely represent ionized and nonionized tyrosinyl residues (6). In contrast, cFLIPL exhibits a blue shift to 330 nm when the protein is incubated in buffer containing intermediate concentrations of urea (Fig. S2, D and E).
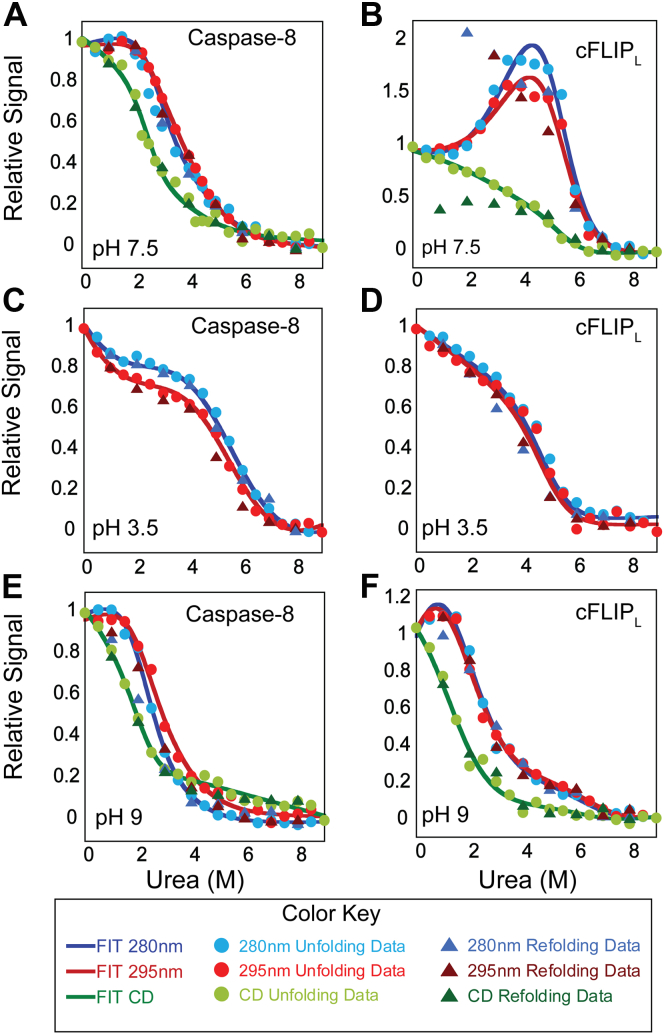
To examine the unfolding of caspase-8 and cFLIPL, we incubated proteins in urea-containing buffer, from 0 M to 9 M urea. Following equilibration, we monitored changes in fluorescence emission (following excitation at 280 nm or 295 nm) to examine changes in tertiary structure, and we monitored changes in far-UV CD to examine changes in secondary structure, as described previously (14). The data for caspase-8 (Fig. 2A) and cFLIPL (Fig. 2B), at pH 7.5, show a pretransition between 0 M and ∼1.5 M urea, where there is little to no change in the signal, followed by a cooperative change in signal between ∼1.5 M and ∼4 M urea. In the cooperative transition, the fluorescence emission decreases relative to the native conformation for caspase-8 but increases in the case of cFLIPL. For caspase-8, the cooperative transition is similar for the three spectroscopic probes (Fig. 2A), except that the loss of secondary structure occurs at lower urea concentrations. In contrast, cFLIPL exhibits a higher fluorescence emission following the first transition (∼4 M urea), and one observes a larger change when the protein is excited at 295 nm compared with excitation at 280 nm (Fig. 2B). A second cooperative transition occurs between ∼4 M and 7 M urea. For both caspase-8 (Fig. 2A) and cFLIPL (Fig. 2B), the protein is largely unfolded at urea concentrations above 7 M. As expected for a monomer, the findings of our studies at two protein concentrations (2 and 6 μM) show that the data were independent of concentration, for both the proteins. Refolding data show that caspase-8 folds reversibly at pH 7.5 (Fig. 2A). In contrast, cFLIPL refolds reversibly at urea concentrations greater than 3 M, but at lower concentrations, the refolding signals did not recapitulate the unfolding signal (Fig. 2B). The lack of reversibility for cFLIPL at lower urea concentrations and at pH 7.5 is discussed in more detail later.
Figure 2.
Equilibrium unfolding of caspase-8 and cFLIPLat pH 3.5, 7.5, and 9. Equilibrium unfolding of caspase-8 (A–C) and cFLIPL (D, E, and F) monitored by fluorescence emission with excitation at 280 nm ( ), 295 (
), 295 ( ), and CD (
), and CD ( ). Refolding of caspase 8 monitored by fluorescence at 280 nm (
). Refolding of caspase 8 monitored by fluorescence at 280 nm ( ), 295 (
), 295 ( ), and CD (
), and CD ( ). As described in the text, solid lines represent global fits to fluorescence emission data at 280 nm (
). As described in the text, solid lines represent global fits to fluorescence emission data at 280 nm ( ), 295 nm (
), 295 nm ( ), and CD (
), and CD ( ). Thermodynamic parameters obtained from the fits are described in Tables S1 and S2.
). Thermodynamic parameters obtained from the fits are described in Tables S1 and S2.
We have shown previously that changes in pH are an excellent perturbant for examining the caspase folding landscape (6, 15, 17). Both the protein conformation and the oligomeric state, in the case of human caspase-3, are sensitive to changes in pH, resulting in changes in protein stability. In order to determine whether the monomeric caspases undergo similar changes, we examined the equilibrium folding/unfolding over a broad pH range, from 3.5 to 9 for caspase-8 and cFLIPL. Although the full range of data are shown in figures for caspase-8 (Fig. S3) and for cFLIPL (Fig. S4), we show results for the lowest (Fig. 2, C and D) and highest (Fig. 2, E and F) pHs for caspase-8 and cFLIPL, respectively, as a comparison to unfolding at pH 7.5 (Fig. 2, A and B). We note that we were unable to obtain data at pH 5.5 for either protein because of protein aggregation. As described later, the far-UV CD signal of caspase-8 and cFLIPL decreased below pH 7 and pH 6, respectively, suggesting a loss of secondary structure. Thus, we monitored only changes in fluorescence emission at pHs below 6.
Collectively, the data were fit to equilibrium folding models that best describe the folding/unfolding over the broad range of pH, and the results are shown as the solid lines in the figures (Figs. 2, S3 and S4). The data for both caspase-8 and cFLIPL were best described by a three-state folding model in which the native protein unfolds through a partially folded intermediate prior to unfolding (N↔I↔U). For caspase-8, at all pHs, the fluorescence emission of the partially folded intermediate is red shifted and has a higher fluorescence emission compared with the native protein (Fig. S2, A and B), demonstrating that the single tryptophan residue is quenched in the native conformation relative to the partially folded intermediate or the unfolded conformation. The data for cFLIPL are best described by a three-state equilibrium folding model between pH 4.5 and 9 (N↔I↔U) and by a two-state folding model (I↔U) below pH 4.5. As described later for both proteins, one or more of the conformational states is sensitive to changes in pH, which affects the relative population of the species during unfolding. In contrast to caspase-8, the fluorescence emission signal of the intermediate state for cFLIPL is blue shifted and has a lower fluorescence emission signal compared with the native protein (Fig. S2, D and E), demonstrating that one or more tryptophans transition to a more hydrophobic environment in the partially unfolded intermediate compared with the native state. Interestingly, the folding/unfolding of cFLIPL is reversible at pH below 6.5 (Fig. 2D) and above 8 (Fig. 2F), but at the pH range closer to neutral pH (pH 6.5–8) (Fig. 2B and S4), folding is irreversible. In contrast, the folding/unfolding of caspase-8 is reversible at all pHs.
Global fitting of equilibrium unfolding data indicates that the stability of the protomer is conserved in all caspases
As described previously (19), the data at each pH for caspase-8 and cFLIPL were fit globally to the folding models described previously in order to determine the conformational free energies and m-values associated with each transition. Results of the fits shown as the solid lines in Figures 2, S3 and S4 are presented in Tables S1 and S2. The free energy and cooperativity index (m-value) for each unfolding transition were estimated by fitting approximately 20 experimental replicates for fluorescence emission at protein concentrations of 2 μM and 6 μM and six replicates for far-UV CD. The data shown in the figures are averages of the replicates.
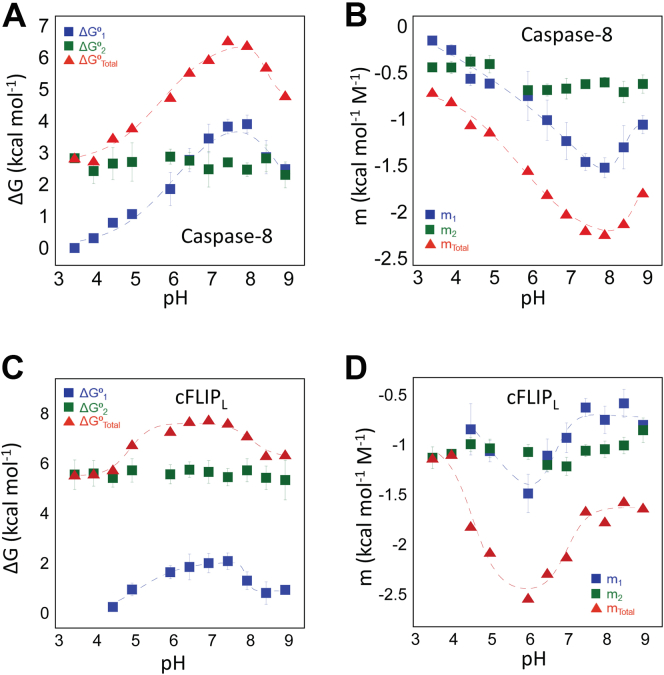
The results for caspase-8 show that, at pH 7.5, the total conformational free energy and m-value are 6.3 kcal mol−1 and 2.1 kcal mol−1 M−1, respectively. Over the pH range of 6.5 to 9, the two transitions exhibit similar free energies in caspase-8, although the first transition (N↔I) has a somewhat higher conformational free energy (ΔG01) compared with the second transition (I-U) (ΔG02), ∼3.7 kcal mol−1 versus 2.6 kcal mol−1 (Table S1), as well as m-values (∼1.5 kcal mol−1 M−1 versus 0.7 kcal mol−1 M−1). The empirical relationship between m-values and surface area described by Scholtz et al. (20) suggests that more hydrophobic surface area is exposed in forming the intermediate conformation than during the unfolding of the intermediate. At lower and higher pH, the conformational free energy of the first transition decreases, whereas that of the second transition remains constant. The change in relative population of the native conformation is observed in the equilibrium folding data (Fig. S3), in that, as the relative population of the native protein decreases, one observes a plateau between ∼4 M and ∼6 M urea, which reflects an increase in the relative population of the intermediate, I. In addition, the midpoint of the transition for N↔I decreases at lower pH. Indeed, at pH 3.5, the pretransition region disappears, so it is difficult to obtain accurate fits to the first transition. Overall, the data obtained from the global fits are shown in Figure 3, A and B and Table S1 for caspase-8 and demonstrate that the protein is maximally stable between pH 7 and 8. At lower and higher pH, the native protein is destabilized relative to the partially folded intermediate, I, such that the relative population of the intermediate increases. Thus, the change in the total conformational free energy versus pH is due to the destabilized native conformation. The changes in m-value versus pH also show the same trend. That is, the decrease in the m-value at lower pH reflects the increased relative population of the partially folded intermediate.
Figure 3.
pH-dependent changes in conformational free energy of caspase-8 and cFLIPL. Conformational free energies of (A) caspase-8 and (C) cFLIPL depicting the experimentally determined free energy values for native state −ΔG⁰1 ( ), intermediate state −ΔG⁰2 (
), intermediate state −ΔG⁰2 ( ), and total free energy −ΔG⁰Total (
), and total free energy −ΔG⁰Total ( ). Experimentally determined m-values for (B) caspase-8 and (D) cFLIPL depicting values for native state -m1 (
). Experimentally determined m-values for (B) caspase-8 and (D) cFLIPL depicting values for native state -m1 ( ), intermediate state -m2 (
), intermediate state -m2 ( ), and total m-value -mtotal (
), and total m-value -mtotal ( ). Dashed lines in A–D represent fits of the data to determine pKa values, as described in the text.
). Dashed lines in A–D represent fits of the data to determine pKa values, as described in the text.
The global fits of the data for cFLIPL demonstrate that, unlike caspase-8, the first transition (N↔I) has a substantially lower conformational free energy (ΔG01) compared with the second transition (I-U) (ΔG02), ∼2.2 kcal mol−1 versus 5.5 kcal mol−1 (Table S2). Together, the data show a substantial increase in the stability of the partially folded intermediate of cFLIPL in comparison to that of caspase-8. Similar to caspase-8, however, the conformational free energy (ΔG01) of first transition (N↔I) exhibits a pH dependence, whereas that of the second transition (ΔG02) is independent of pH (Table S2 and Fig. 3, C and D). Altogether, the data for folding/unfolding from pH 3.5 to 9 show that both caspase-8 and cFLIPL are sensitive to pH changes, similar to the effector caspase dimers (6, 14), because of destabilizing the native conformation relative to a partially folded intermediate. The higher m-values for the N↔I transition in caspase-8 indicate a larger exposure of hydrophobic surface area compared with the same transition for cFLIPL, whereas the m-values for the second transition (I↔U) are higher for cFLIPL indicating a more compact conformation for the intermediate state in cFLIPL. The protomers of caspase-8 and cFLIPL exhibit a ΔG0conf of 6 to 8 kcal mol−1, which is comparable to the monomeric intermediate observed in the equilibrium unfolding of executioner caspases-3 and -7 as well as that of the common ancestor of effector caspase dimers (6, 14). In those cases, the ΔG0conf of the monomeric folding intermediate was determined to be ∼5 to 7 kcal mol−1 at pH 7.5 and 25 °C.
Using the values acquired from the global fits of the equilibrium unfolding data and the cooperativity indices determined for each transition (Tables S1 and S2), we calculated the equilibrium distribution of species (N, I, and U) for each protein at each pH and throughout the urea concentration range of 0 to 9 M, as described previously (17). The data are shown in Fig. 5 (caspase-8) and Fig. S6 (cFLIPL). For caspase-8, at pH >6.5, the native species (N) is well populated at low urea concentrations, from 0 to 2 M, and the intermediate species (I) shows a maximum population at ∼3 M urea. The unfolded fraction is well populated above 5 M urea (Fig. S5). At pH <6.5, one observes an increase in the population of the folding intermediate, I, in the absence of urea, such that at pH 3.5, the “native” protein is an ensemble of native (N) and intermediate (I) conformations. Similar results are observed for cFLIPL in that the fraction of native species (N) decreases relative to the folding intermediate, I, in the absence of urea (Fig. S6). For cFLIPL, however, the native conformation is not well populated below pH 4.5.
We note that the thermodynamic parameters (ΔG01 and m1) reported for the first transition (N↔I) for cFLIPL, from pH 6 to pH 8.5, are uncertain because of hysteresis. From pH 6 to pH 8.5, the fraction of the intermediate state is greatest at 4 M urea (Fig. S6), and the folding is shown to be reversible up to 4 M urea (Fig. S4). Furthermore, at pH 4 and 4.5, the intermediate is the predominant fraction in the absence of urea, and since the folding is completely reversible under those conditions, the complete dataset at all pH suggest that the thermodynamic parameters (ΔG02 and m2) reported for the second transition (I↔U) are accurate. Given that trends in the data indicate that the monomeric structure has a conformational free energy of 5 to 7 kcal mol−1, in the entire family of caspases, the conformational free energy of the first transition (ΔG01), should fall within the reported range, resulting in ∼7 to 8 kcal mol−1 of total conformational free energy from pH 6 to pH 8.5 (Table S2 and Fig. 3, C and D). However, we note that without full reversibility under conditions of pH 6 to 8.5, the true values of ΔG01 and m1 for cFLIPL cannot be accurately determined.
Caspase-8 and cFLIPL undergo pH-dependent conformational changes
The effector caspase dimers have previously been shown to undergo a pH-induced conformational change that results in an enzymatically inactive dimeric conformation, with a pKa ∼6 for the transition (6, 17). Based on the data described previously for caspase-8 and cFLIPL, where we showed that the native conformation is sensitive to changes in pH, we examined changes in far-UV CD and fluorescence emission signals for the native protein versus pH, and we examined the midpoint of the folding transitions that are sensitive to pH changes, namely N↔I (ΔG01) (Fig. S7). We note that the raw CD data show a decrease in signal below pH 6, suggesting a loss in secondary structure (Fig. S7, A and B). At the same time, the fluorescence emission data suggest that the proteins retain buried aromatic residues. While the data are consistent with the unfolding of the small subunit (described later), the fluorescence emission data suggest aromatic residues that remain buried until the protein is incubated in urea. Although the interpretation of the CD spectra is less clear, the loss of signal at 230 nm and the appearance of minima at 240 nm may also be due to clusters of aromatic residues, particularly phenylalanine and tyrosine residues. The large subunit of caspase-8 contains 15 Phe/Tyr residues, whereas the small subunit contains only seven Phe/Tyr residues. Altogether, the data indicate that, at low pH, the protein has an unfolded small subunit and little to no secondary structure in the remaining conformational state. The structure that remains is presumably contributed by the large subunit, but the remaining structure appears to contain clusters with partially buried aromatic residues.
When analyzing the entire pH profile, one observes that that caspase-8 exhibits a maximum far-UV CD signal between pH 7 and 8, whereas cFLIPL exhibits a maximum far-UV CD signal between pH 6 and 7 (Fig. S7, C and D), which is consistent with the pH range determined for maximum conformational stability (Fig. 3) for caspase-8 (pH 7–8) and cFLIPL (pH 6–7). As described previously, we also determined the midpoints for the transitions from examining changes in the fraction of species, which again shows the effects of pH on the native conformation (N) but not the folding intermediate (I), for caspase-8 (Fig. S7E) and cFLIPL (Fig. S7F).
As described previously (21), we fit the pH-dependent transitions for the change in secondary structure (Fig. S7, C and D), transition midpoints (Fig. S7, E and F), and conformational free energies and m-values (Fig. 3, A–D) to determine the pKa for the conformational change, and the fits are shown as the dashed lines in the figures. The results are reported in Table S3 (caspase-8) and Table S4 (cFLIPL). Overall, the analysis shows two pH-dependent transitions, with pKa1 ∼5.6 and pKa2 ∼8.1, and little variation between the two proteins. We suggest that the variety of different probes, such as secondary structure, conformational free energy, and m-values, and transition midpoints, likely report on the same conformational changes, regardless of the minor variations in the individual pKa values. In comparison to the dimeric effector caspases, the first transition is conserved, with pKa ∼6, whereas the second transition, with pKa ∼8.1, may be unique to the monomeric caspases. Thus, it appears that the caspase protomer undergoes a pH-dependent transition, regardless of oligomeric state. In the effector caspase dimer, the transition results in reversible formation of an enzymatically inactive intermediate (6, 14), whereas the monomeric caspases partially unfold. We note that in caspase-8, the pH-dependent formation of the folding intermediate, I, is reversible, whereas in cFLIPL, the transition to I is not reversible at pHs close to physiological pH (see Fig. S4 and described previously). Because the first transition occurs in the monomer and dimer subfamilies, our data suggest that the property is inherent in the caspase protomer, and that the mechanism is conserved.
MD simulations in the presence of urea reveal the small subunit and helix 2 are unstable
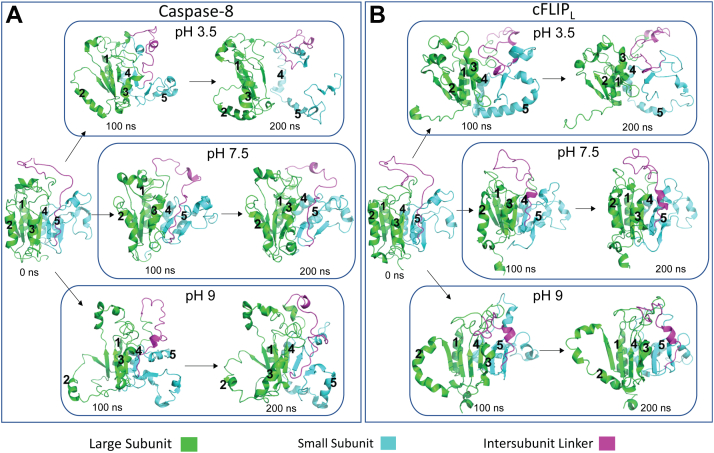
In order to further examine conformational changes in the caspase protomers, we performed MD simulations for 200 ns using caspase-8 and cFLIPL. The starting structures were modeled using the solution structure of the protease domain of procaspase-8 (Protein Data Bank [PDB] ID: 2K7Z) (22). As described in the Experimental procedures section, loop regions that connect β-strand 5 with active site loop 3 and α-helix 5 with β-strand 6 (Fig. S1A) were absent in the solution structure. The missing residues were modeled using SWISS-MODEL to produce a structure for the protomer of caspase-8 with contiguous sequence connectivity. The resulting model of the caspase-8 protomer was then used to generate the starting structure for the protomer of cFLIPL. The solution structure of procaspase-8 provides a view of the protomer prior to oligomerization and substrate binding because structures of the caspase-8 homodimer and of the caspase-8–cFLIPL heterodimer, solved by X-ray crystallography, contain inhibitor bound to the caspase-8 active site. In the model used for MD simulations, the intact IL prevents proper active site formation (Fig. 4).
Figure 4.
Molecular dynamics simulations. Of caspase-8 (A) and of cFLIPL (B) in 5 M urea at three different protonation states representing pH 3.5, 7.5, and 9. For A and B, time points of 0, 100, and 200 ns are shown. The large subunits and small subunits of caspase-8 and cFLIPL, are shown in green and cyan, whereas the intersubunit linker is shown in magenta (refer color key inset). Helices 1 (back), 2 (front), and 3 (front) on the surface of the large subunit and helices 4 (back) and 5 (back) on the small subunit are labeled.
For each protein, the simulations were performed in the presence and absence of 5 M urea and at pH 3.5, 7.5, and 9. Representative time frames of 0, 100, and 200 ns are shown in Figure 4 for both proteins. The data show that the small subunit unfolds because of helices 4 and 5 lifting away from the β-sheet. The unfolding of the helix 4/5 unit then pulls β-strand 6 away from the core such that the core structure of β2-1-3 (large subunit) remains intact, but the small subunit is largely unfolded. Within the large subunit, helices 2 and 3 separate from the β-sheet core and expose the core to solvent. Although similar processes occur at all pHs and for both proteins, one observes greater unfolding at pH 3.5 and pH 9 compared with pH 7.5.
We examined the unfolding of the proteins by monitoring the root mean square fluctuation (RMSF) of each amino and compared the results at each pH and in the presence and absence of urea for caspase-8 (Fig. S8, A–C) and cFLIPL (Fig. S9, A–C). The RMSF varies with protonation states at pH 3.5, 7.5, and 9 for both caspase-8 and cFLIPL, correlating with the extent of unfolding shown in Figure 4. In order to compare the changes in RMSF because of the presence of urea, we first subtracted the RMSF of simulations in the absence of urea from the RMSF in the presence of urea for caspase-8 (Fig. S8, D–F) and cFLIPL (Fig. S9, D–F). At pH 7.5, one observes an increase in RMSF of surface helices in the presence of urea. At pH 9, one observes an increase in the RMSF of helices 4 and 5 in the small subunit, whereas an increase in RMSF is observed throughout the protein at pH 3.5. We transformed the ΔRMSF values into B-factors and mapped them onto the structure to provide a visual representation of the unfolded regions on the structure. For both proteins, at pH 7.5 and in 5 M urea, helices 3 and 4 and the connecting loop are destabilized (Fig. S8G [caspase-8] and Fig. S9G [cFLIPL]), and the fluctuations increase at lower and higher pH (Fig. S8, H and I [caspase-8] and Fig. S9, H and I [cFLIPL]). In addition, at lower and higher pHs, the surface helices, particularly helices 2 and 3, show increased fluctuations leading to unfolding. Together, the data from MD simulations show that the small subunit unfolds first and that a higher degree of unfolding occurs at pH 3.5 compared with higher pH.
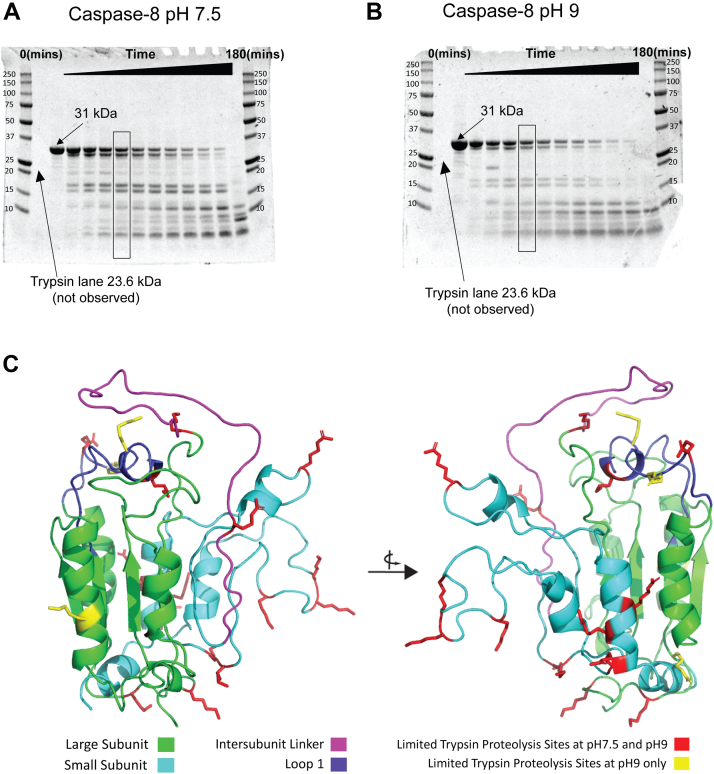
Limited trypsin proteolysis of caspase-8 confirms that the small subunit is less stable than the large subunit
As shown by equilibrium unfolding experiments (Fig. 3), the native conformation of caspase-8 is most stable at pH 7 to 8, and it is destabilized at both higher and lower pH. In order to further examine changes in the protein conformation versus pH, we performed limited trypsin proteolysis of caspase-8 at pH 7.5 and 9. Trypsin activity curves from pH 7 to pH 9 display a comparable catalytic efficiency at pH 7 and 9, indicating that the activity is not substantially different, allowing us to examine pH-induced caspase-8 conformational changes (23). As described in the Experimental procedures section, caspase-8 was treated with trypsin at pH 7.5 or at pH 9. Samples were collected at 15 min time intervals until 2 h, followed by two 30 min intervals between 2 h and 3 h, and finally, a sample was collected after incubation overnight. Protein fragments were separated by SDS-PAGE (Fig. 5, A and B). We note that the trypsin enzyme was not observed on the gel since the concentration of trypsin was low compared with that of caspase-8. We analyzed the changes in intensity of the native band at pH 7.5 and 9, and then we fitted a single exponential equation to the data (Fig. S10, A and B). The apparent rate constant that we obtained from this analysis is displayed in Fig. S10C, and it reveals that the full-length protein (31 kDa) is cleaved with a half-time of 20 min at pH 9, in contrast to a half-time of 40 min at pH 7.5. This indicates that the enzyme is less stable at pH 9, which is consistent with our conformational free energy estimates (Fig. 3A), which demonstrate a decrease in free energy values.
Figure 5.
Limited trypsin proteolysis of caspase-8 at pH 7.5 and 9.A, results of cleavage of caspase-8 (31 kDa) at pH 7.5. B, results of cleavage of caspase-8 (31 kDa) at pH 9. Sizes of fragments are labeled. For A and B, molecular weight markers are shown in the first and last lanes, and O/N refers to samples incubated overnight. The fifth lane that refers to the products analyzed is boxed. C, cleavage products of trypsin proteolysis at pH 7.5 and 9, respectively, were determined by mass spectrometry as described in the text and represented on the structure of caspase-8 (Protein Data Bank ID: 2K7Z). The small subunit is depicted in cyan, whereas the large subunit is depicted in green. Cleavage sites at both pH 7.5 and 9 are shown by red side chains, whereas cleavage products at pH 9 are represented by yellow side chains. The intersubunit linker is depicted in magenta, whereas loop 1 is indicated in purple.
In independent studies, we treated caspase-8 with trypsin for 60 min at pH 7.5 and 9 (representing the fifth lane from the 0 time point lane in Figure 5, A and B) and performed MALDI-TOF analysis to quantify the molecular weights of all the fragments. We used data from the 60 min time because they yielded the greatest number of distinct products on the gel (Fig. 5, A and B). To gain insight into the regions that are susceptible to the highest cleavages, and hence represent the regions that are destabilized, we examined fragments with the highest intensities at pH 7.5 and 9 (Fig. S11). All the cleavage sites were determined (Fig. S12) and were mapped onto the structure of caspase-8 (Fig. 5C) to illustrate that the largest contributions to the fragments in Figure 5, A and B are the result of cleavages occurring on the IL, small subunit, and large subunit. Cleavages in the small subunit account for nine of the 15 identified sites, with cleavages on active site loop 3 (one site), helix 4 (three sites), active site loop 4 (three sites), and C terminus (two sites), whereas cleavages in the large subunit are limited to the N terminus (two sites) and active site loop 1 (two sites). At pH 9, additional cleavage sites are observed in active site loop 1 (two sites), helix 1 (one site), and the loop region between β-sheet 1 and the N terminus (Fig. 5C). These data suggest that the area surrounding helix 2 is destabilized, which reduces the stability of the large subunit and hence the molecule at pH 9, resulting in a higher rate of cleavage at this pH. In addition, two high-intensity cleavage products of 4232 and 4504 Da (Fig. S12) that map to helix 2 are consistent with the observations in MD simulations that suggest the large subunit is destabilized around helix 2 (Fig. 4A).
The data show that several regions of the small subunit are accessible to cleavage by trypsin at both pH, whereas cleavage in the large subunit is more limited to the N terminus and active site loop 1. Taken together, the results of limited trypsin proteolysis are consistent with the results of the MD simulations that show that the small subunit is less stable and thus more accessible to cleavage by trypsin compared with the large subunit. In addition, the increased cleavage at pH 9 of helix 2, the loop linking the N terminus to β-sheet 1, and active site loop 1 on the large subunit is consistent with the fluctuations of the large subunit observed in MD simulations.
Discussion
Molecular evolution has broadened a limited set of ancient protein folds to perform varied biochemical tasks in a multitude of present-day species (24). The caspase family of enzymes has evolved into multiple members in several subfamilies, with new functions and allosteric regulation, while preserving the fold of a ∼4 billion-year-old ancestral scaffold (8). We show here that caspase-8 and cFLIPL unfold at pH 7.5 by a three-state equilibrium folding pathway, where the native protein (N) unfolds to a partially folded intermediate (I) prior to unfolding (U). The overall conformational free energies of 6.2 kcal mol−1 (caspase-8) and of 7.7 kcal mol−1 (cFLIPL) are similar to that determined previously for a monomeric folding intermediate of dimeric effector caspases, which is in the range of 5 to 7.0 kcal mol−1 (6, 14). In general, both caspase-8 and cFLIPL show similar properties, even though they are separated by ∼300 million years of evolution from each other (25) and nearly 650 million years removed from the effector subfamily (6). Indeed, cFLIPL evolved to be a pseudoenzyme that plays an important role in necroptosis through forming a heterodimer with caspase-8, whereas caspase-8 evolved as an activator of effector caspases (12). The similar conformational free energies of the monomers suggest that the folding landscape has been conserved throughout evolution, even as the two subfamilies evolved into multiple members with different substrate specificities and allosteric regulation.
The characterization of the native protomer of caspase-8 and cFLIPL is consistent with the monomeric intermediate described previously for dimeric effector caspases. For example, the native conformation of the caspase-8 and the cFLIPL protomer has a partially disordered active site, as observed by limited trypsin proteolysis, yet the tryptophan residue near the active site is buried. In addition, the m-values for equilibrium folding demonstrate that the monomer is only partially folded. For example, Scholtz et al. (20) described a correlation between unfolding m-values, the change in buried accessible surface area, and number of residues. For all caspases studied to date, the m-values for unfolding of the monomer range from 1.20 to 1.98 kcal mol−1 M−1 (Tables S1 and S2) (6, 14, 15). Using the equations described by Scholtz, the change in buried accessible surface area for unfolding the caspase protomer is ∼8000 to 15,000 Å2, corresponding to ∼96 to 170 residues. Caspase protomers comprise ∼260 amino acids, so the collective equilibrium folding data suggest that the “native” protomer contains substantial surface area exposed in loops and other unstructured regions. Our data, shown here, from MD simulations and limited trypsin proteolysis suggest that the loss of buried surface area results from fluctuations in the small subunit and the surface helices.
We note that the assembly of the dimer of effector caspases does not occur through assembly of two preformed “native” protomers because an additional conformational change occurs after dimerization that involves active-site loop rearrangements (14, 21, 26). The flexibility of the small subunit within the protomer likely results in a slow rate of dimerization. We showed previously that the rate of dimerization for procaspase-3 is ∼70 M−1 s−1, which is very slow compared with many homodimers (27). Procaspase-8 has been shown to form homodimers in the presence of high concentrations of kosmotropes, such as sodium citrate at 1 M (28). Extrapolation of the measured dimerization rates to the absence of kosmotrope, however, suggested a second-order rate of dimerization near zero. Decreasing the slow rate of dimerization by a small factor would essentially trap the protomer as a monomer, which shows the importance of the DED motifs and the activating platforms for increasing the local concentration of protomer to facilitate dimerization. Indeed, it was suggested that negative design elements in the dimer interface may decrease the rate of dimerization while simultaneously increase specificity (27). For example, in the case of caspase-8, F468, on β-strand 6 of one protomer, interacts with P466 in the interface of the second protomer, resulting in intersubunit stacking interactions. In the caspase-3 dimer, introduction of the V266H variant in the dimer interface resulted in a kinetically trapped monomer that slowly dimerized following rearrangement of the bulky histidine residues (29), demonstrating that reducing the very slow rate of dimerization effectively traps the protomer. We showed that optimizing the dimer interface by replacing P466 and F468 in caspase-8 increased the rate of dimerization but did not result in a stable dimer, suggesting that additional factors are required for dimerization aside from an optimized β-strand 6 (27). The data presented here suggest that a key factor resulting from dimerization is the stabilization of the small subunit. Combined with our previous kinetic folding data for procaspase-3 (29), where we showed that the monomer forms a dimerization-competent species as well as other species that are not competent to form dimers, we suggest that the fluctuations in the small subunit result in an ensemble of protomer conformations, which reduces the concentration of a dimerization-competent conformation and effectively decreases the second-order rate of dimerization. Thus, the evolution of monomer and dimer caspase subfamilies appears to be a result of tailoring the conformational dynamics of the protomer. Evolutionarily, dimers may have originated as a consequence of stabilizing the small subunit, where a modest increase in the rate of dimerization would provide access to additional regions of the conformational landscape, that of the dimer, leading to substantial increases in conformational free energies of the dimer versus the monomer.
Different cellular compartments have been shown to have altered pH environments. The pH of cytosol, endoplasmic reticulum, and nucleus is 7.2, mitochondria are pH 8, the golgi network is pH 6.5 to 6, and lysosomes are pH 4.7 (30). Our findings reveal that changes in the pH of the environment can fine-tune the stability and hence the conformational landscape of these enzymes. Localizing caspase-8 and cFLIPL to different compartments could be a strategy used by cells to fine-tune conformational dynamics and affect certain pathways. In solid tumors with pH dysregulation, for example, the initiator caspase-8 and cFLIPL can be substantially destabilized, affecting both apoptosis and necroptosis pathways (31).
It is worth noting that caspase conformations are finely regulated by dimerization, metal binding, post-translational modifications, and limited proteolytic cleavages (5). Most post-translational modifications occur in the large subunit, with just two sites known in the small subunit of caspase-8 (Fig. S1). An evolutionarily conserved allosteric site is located at the base of helices 2 and 3 and near the residues in the N and C termini (32). Several post-translationally modified amino acids are known to be localized near the conserved allosteric site. For example, S150 and T152 in caspase-3, S347 in caspase-8, and T173 in caspase-7 are located at the base of helix 2, and their phosphorylation by p38 mitogen-activated protein kinase or by p21-activated kinase 2 decreases caspase activity (32, 33, 34). Our folding studies, presented here, demonstrate that the native state of caspase-8 and cFLIPL can be modulated by protonation/deprotonation through changes in pH. Results from our MD simulations show that helices 2 and 3 of the large subunit are unstable, suggesting that allosteric modulation, through ligand binding to the allosteric site and post-translational modifications, result in changes to the conformational ensemble to favor the partially folded conformation (I) by destabilizing the two surface helices near the allosteric site. Interestingly, all caspases have shown a pH-dependent conformational change, with pKa ∼6.
Experimental procedures
Cloning, protein expression, and protein purification
The plasmids for caspase-8 (35) and cFLIPL (18) were obtained from the Addgene plasmid repository, and the catalytic cysteine was changed to alanine using site-directed mutagenesis. The purification steps were described previously (27). The concentration of caspase-8 was estimated using ε280 = 23,380 M−1 cm−1. cFLIPL was purified as described previously (28), and the protein concentration was measured using ε280 = 34,380 M−1 cm−1. We used constructs of caspase-8 and cFLIPL where the prodomain was removed, since the prodomain has been shown to reduce solubility (27). In addition, to prevent autoprocessing of caspase-8, we mutated the catalytic cysteine to alanine. Previous studies have shown that removal of the DED motifs in the prodomain does not affect formation of the active homodimer, so the protein folds correctly in the absence of the prodomain (22).
Sample preparation for equilibrium folding/unfolding
Folding/unfolding experiments were performed as described previously (19). Briefly, stock solutions of urea (10 M), citrate buffer (50 mM sodium citrate/citric acid, pH 3.5–5.5, 1 mM DTT), phosphate buffer (pH 6–8, 1 mM DTT), Tris buffer (50 mM Tris–HCl, pH 8.5–9, 1 mM DTT) were prepared as described (6, 14, 19). For unfolding studies, protein samples were prepared in buffer with urea concentrations ranging from 0 to 9 M. The buffers used to prepare protein and urea solutions provided a range of pH, from 3.5 to 9, as shown in the figures. For renaturation experiments, stock protein was first incubated for 3 h at 25 °C in an 8 M urea-containing buffer. The unfolded protein was then diluted into the appropriate buffer with urea concentrations ranging from 1 to 8 M utilized. For all equilibrium folding/unfolding studies, the final protein concentration ranged from 2 to 6 μM. In both denaturation and renaturation studies, samples were incubated for a minimum of 16 h at 25 °C.
Fluorescence emission and CD measurements
Fluorescence emission was acquired using a PTI C-61 spectrofluorometer (Photon Technology International) from 300 to 400 nm following excitation at 280 or 295 nm. Excitation at 280 nm follows tyrosinyl and tryptophanyl fluorescence emission, whereas excitation at 295 nm follows the tryptophanyl fluorescence emission. Experiments on folding/unfolding were carried out as previously described (6, 14, 19). In brief, stock solutions of urea (10 M), citrate buffer (50 mM sodium citrate/citric acid, pH 3.5–5.5, 1 mM DTT), phosphate buffer (pH 6–8, 1 mM DTT), and Tris buffer (50 mM Tris–HCl, pH 8.5–9, 1 mM DTT) were made as indicated (19). Protein samples were incubated in buffer containing urea concentrations ranging from 0 to 9 M for unfolding and at final pHs shown in the figures. For refolding, stock protein was incubated for 3 h at 25 °C in buffer containing 8 M urea. The unfolded protein was then diluted into the appropriate buffer with urea concentrations ranging from 1 to 8 M. The final protein concentration ranged from 2 to 6 μM for all equilibrium folding/unfolding investigations. Samples were incubated at 25 °C for at least 16 h in both denaturation and renaturation tests. CD measurements were recorded using a J-1500 spectropolarimeter (Jasco) between 220 and 240 nm. Fluorescence and CD spectra were measured using a 1 cm path length cuvette and constant temperature (25 °C). All data were corrected for buffer background.
Data analysis and global fits of equilibrium folding/unfolding data
The data were fit globally and interpreted as described previously (19). Briefly, fluorescence emission and CD data were collected between pH 3.5 and 9 for both proteins and at two protein concentrations (2 and 6 μM), which resulted in 15 different datasets at each pH. The data were fit to a two-state or three-state equilibrium folding model, as described later. The folding data for caspase-8 show a change in slope at ∼3 M urea from pH 6.5 to pH 9, which is much more pronounced with the CD signal. The midpoint of this slope shifts to lower urea concentration from pH 5 to 3.5, requiring a three-state model for fitting (Fig. S3). Therefore, for caspase-8, at all pHs, the data were best fit to a three-state equilibrium folding model (Equation 1), where the native conformation unfolds to a partially folded intermediate (I) before unfolding (U).
| (1) |
For cFLIPL, the data were best-fit to three-state equilibrium folding model between pH 4.5 and pH 9 (Equation 1) since a simple two-state model cannot account for the change in slope between 3 M to 4 M urea. Below pH 4.5, the data for cFLIPL were best fit to a two-state model, as described by Equation 2, in which the native conformation (N) is in equilibrium with the unfolded state (U).
| (2) |
Both folding models have been described in detail previously (19). Global fits of the equilibrium folding/unfolding data for both proteins and at each pH were performed in Igor Pro (WaveMetrics, Inc) using the appropriate folding model from Equations 1 or 2, as reported earlier (6, 14, 19).The results of the global fits are shown as the solid lines in the figures, and ΔG0 and m-values obtained from the fits are provided in Tables S1 and S2. We note that two species are distinguished by their differences in intrinsic fluorescence and that the intrinsic fluorescence of each individual species is assumed to be similar at all pHs, such that the change in fluorescence emission is due to a change in the relative population of each species. With those caveats, the relative population of the native and intermediate species versus urea concentration are estimated based on the values of ΔG and m (Figs. S5 and S6). Thus, from the global fits, one can determine the fraction of species, which has been described in detail for a two-state and three-state equilibrium model (19). Under certain conditions, the two species, N and I, are both populated in the absence of urea because of the low stability of the native conformation relative to the intermediate.
To determine pKa values for pH-dependent transitions, the data were fit as described previously (21, 36). The results of the fits are shown as the dashed lines in the figures, and pKa values obtained from the fits are provided in Tables S3 and S4 and are described in the text.
MD simulations with and without urea
We modeled monomeric structures of caspase-8 and cFLIPL using the NMR structure of caspase-8 (PDB ID: 2K7Z). Since most of the caspase structures in the PDB represent crystal structures of active caspases, modeling programs, such as AlphaFold, tend to model the protein according to the enzymatically active conformation (not shown). Since we examined the monomeric conformation rather than the active dimer, we elected to use the solution structure of caspase-8, which we suggest is more representative of the monomer, to model the caspase structures. Loop regions missing in the NMR structure of caspase-8 (PDB ID: 2K7Z) were modeled on Swiss-Model using the template of the NMR structure (37). The H++ server was used to protonate structures at pH 3.5 and 9, the salinity was 0.15 M, the internal dielectric was 10, and the external dielectric was 80. The orientation correction of asparagine, glutamine, and histidine groups based on van der Waals contacts and H-bonding was turned on (38). The urea molecule was created using Avogadro, as described (39). The PDB file of the urea molecule is attached to the supporting information. Force field parameters for the simulations in urea were adopted from Smith et al. (40). The ratio of water and urea molecules added to the system to obtain a concentration of 5 M urea was calculated as described (41). Gromacs MD package was used to perform MD simulations (42). Briefly, SPC water molecules were replaced with 560 molecules of urea (41). The structure of cFLIPL (PDB ID: 3H11) was solved previously by X-ray crystallography (43) and shows the protein was cleaved in the IL and is in a dimer-competent conformation. Thus, we utilized the NMR structure of caspase-8 described previously to model the cFLIPL protomer, also using Swiss-Model as described for caspase-8. The protein was placed in a cubic box of 6 × 6 × 6 nm3 and dissolved either in SPC water or the previously described urea solution. Six sodium ions were used to neutralize the caspase-8 system, whereas one chloride ion was used to neutralize the cFLIPL system. Constraints on the positions of all heavy atoms were used for all equilibration runs. The system was then minimized using the steepest-descent method down to an Fmax (maximum force) >1000 kJ mol−1 nm−1. NVT (constant temperature and volume) and NPT (constant temperature and pressure) equilibration was carried out for 100 ps using leap frog integrator every 2 fs for 50,000 steps. Bond angles and lengths were constrained using LINCS algorithm, and the vervet cutoff scheme of 1 nm was used for both electrostatics and van der Waals forces. Temperature was maintained at 300 K with a coupling constant of 0.1 ps using the V-rescale thermostats in NVT and NPT, and equilibration was performed with a coupling constant of 0.1 ps for temperature. In NPT, equilibration pressure was kept constant at 1 bar using Parrinello–Rahman pressure coupling with a 2 ps constant. The Coulomb cutoff distance was 1 nm, the Lennard–Jones cutoff distance was 1 nm, the Fast Fourier Transform grid maximum spacing was 0.16 nm, and the interpolation order was cubic (42, 44). Using the final step of the NPT ensemble, a 200 ns production run was carried out, bond angles and lengths were constrained using LINCS algorithm, and the vervet cutoff scheme of 0.9 nm was used for both electrostatics and van der Waals forces. Temperature was maintained at 300 K with a coupling constant of 0.5 ps using the Nose–Hoover algorithm, and pressure was maintained at 1 bar with a coupling constant of 1 ps using Parrinello–Rahman thermostat. The Coulomb cutoff distance was 0.9 nm, the Lennard–Jones cutoff distance was 0.9 nm, the Fast Fourier Transform grid maximum spacing was 0.12 nm, and the interpolation order was cubic (42, 44).
Limited proteolysis coupled with mass spectrometry
Caspase-8 (6 μM) was incubated overnight at 25 °C in a buffer of 50 mM phosphate, pH 7.5, or 50 mM Tris–HCl buffer, pH 9, with 1 mM DTT. Trypsin (0.15 ng) (New England Biolabs) was added following the removal of an aliquot at the zero time point, and the reaction tube was incubated on a revolving mixer (45). Aliquots were removed every 15 min, and the reaction was stopped by adding SDS and incubating at 100 °C for 5 min. Samples were visualized by SDS-PAGE (4–20% gradient gel, Sure Page Gels; GenScript), and band intensity was determined using Image Lab (Bio-Rad). The data for band intensity versus time were fit to single exponential equations using Kaleidagraph (Synergy Software).
The samples from the limited proteolysis were prepared for mass spectrometry using Zip tips to remove salts and impurities. On the plate, 1 μl of sample and 1 μl of matrix were spotted and crystallized. The sinnapinic acid matrix was used to analyze molecular weight over 8 kDa and α-cyano-4-hydroxycinnamic acid matrix was utilized below 6 kDa (46). Samples were analyzed by MALDI-MS (AXIMA Assurance) in linear mode. The cleavage locations were determined using MS-digest on Protein Prospector software, version 6.4.2 (available at: https://prospector.ucsf.edu/prospector) (47, 48). Results were mapped onto the modeled structure of caspase-8 using PyMOL (Schrodinger) molecular visualization system.
Conservation analysis
A total of 1000 sequences of caspases-3, -6, -7, -8, -10 and cFLIPL were obtained from caspbase (https://caspbase.uta.edu/) or National Center for Biotechnology Information and trimmed to have equal representations from each taxon in the chordate lineage resulting in 200 sequences (49). Site-specific conservation was determined using the ConSurf server (https://consurf.tau.ac.il/consurf-old.php) and to map conservation as B-factors onto structures (50). Results were viewed in Jalview and subsequently in PyMOL to generate figures (51).
Data availability
All data are contained in the article and supporting information.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
M. N. and A. C. C. conceptualization; M. N. and A. C. C. methodology; M. N. investigation; M. N. and A. C. C. writing–review & editing; A. C. C. supervision.
Funding and additional information
This work was supported by a grant from the National Institutes of Health (grant number: GM127654 [to A. C. C.].
Reviewed by members of the JBC Editorial Board. Edited by Joseph Jez
Supporting information
References
- 1.van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 3.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anson F., Thayumanavan S., Hardy J.A. Exogenous introduction of initiator and executioner caspases results in different apoptotic outcomes. JACS Au. 2021;1:1240–1256. doi: 10.1021/jacsau.1c00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark A.C. Caspase allostery and conformational selection. Chem. Rev. 2016;116:6666–6706. doi: 10.1021/acs.chemrev.5b00540. [DOI] [PubMed] [Google Scholar]
- 6.Shrestha S., Clark A.C. Evolution of the folding landscape of effector caspases. J. Biol. Chem. 2021;297:1–12. doi: 10.1016/j.jbc.2021.101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medvedev K.E., Kinch L.N., Dustin Schaeffer R., Pei J., Grishin N.V. A fifth of the protein world: Rossmann-like proteins as an evolutionarily successful structural unit. J. Mol. Biol. 2021;433:1–25. doi: 10.1016/j.jmb.2020.166788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ameisen J.C. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002;9:367–393. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- 9.Pop C., Fitzgerald P., Green D.R., Salvesen G.S. Role of proteolysis in caspase-8 activation and stabilization. Biochemistry. 2007;46:4398–4407. doi: 10.1021/bi602623b. [DOI] [PubMed] [Google Scholar]
- 10.Walters J., Pop C., Scott F.L., Drag M., Swartz P., Mattos C., et al. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem. J. 2009;424:335–345. doi: 10.1042/BJ20090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillert L.K., Ivanisenko N.V., Busse D., Espe J., König C., Sergey P.E., et al. Dissecting DISC regulation via pharmacological targeting of caspase-8/c-Cflipl heterodimer. Cell Death Differ. 2020;27:2117–2130. doi: 10.1038/s41418-020-0489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchiya Y., Nakabayashi O., Nakano H., Lemarié A. FLIP the switch: regulation of apoptosis and necroptosis by Cflip. Int. J. Mol. Sci. 2015;16:30321–30341. doi: 10.3390/ijms161226232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aravind L., Koonin E.V. Classification of the caspase–hemoglobinase fold: detection of new families and implications for the origin of the eukaryotic separins. Proteins. 2002;46:355–367. doi: 10.1002/prot.10060. [DOI] [PubMed] [Google Scholar]
- 14.Bose K., Clark A.C. Dimeric procaspase-3 unfolds via a four-state equilibrium process. Biochemistry. 2001;40:14236–14242. doi: 10.1021/bi0110387. [DOI] [PubMed] [Google Scholar]
- 15.Yao L., Clark A.C. Comparing the folding landscapes of evolutionarily divergent procaspase-3. Biosci. Rep. 2022;42:1–13. doi: 10.1042/BSR20220119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinshpon R.D., Shrestha S., Titus-Mcquillan J., Hamilton P.T., Swartz P.D., Clark A.C. Resurrection of ancestral effector caspases identifies novel networks for evolution of substrate specificity. Biochem. J. 2019;476:3475–3492. doi: 10.1042/BCJ20190625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bose K., Clark A.C. pH effects on the stability and dimerization of procaspase-3. Protein Sci. 2005;14:24–36. doi: 10.1110/ps.041003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boatright K.M., Deis C., Denault J.-B., Sutherlin D.P., Salvesen G.S. Activation of caspases-8 and-10 by FLIPL. Biochem. J. 2004;382:651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walters J., Milam S.L., Clark A.C. Practical approaches to protein folding and assembly: spectroscopic strategies in thermodynamics and kinetics. Methods Enzymol. 2009;455:1–39. doi: 10.1016/S0076-6879(08)04201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholtz J.M., Grimsley G.R., Pace C.N. Solvent denaturation of proteins and interpretations of the m value. Methods Enzymol. 2009;466:549–565. doi: 10.1016/S0076-6879(09)66023-7. [DOI] [PubMed] [Google Scholar]
- 21.Bose K., Pop C., Feeney B., Clark A.C. An uncleavable procaspase-3 mutant has a lower catalytic efficiency but an active site similar to that of mature caspase-3. Biochemistry. 2003;42:12298–12310. doi: 10.1021/bi034998x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller N., Mareš J., Zerbe O., Grütter M.G. Structural and biochemical studies on procaspase-8: new insights on initiator caspase activation. Structure. 2009;17:438–448. doi: 10.1016/j.str.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Crewther W.C. The effect of pH and cations on the thermal denaturation of trypsin. Aust. J. Biol. Sci. 1953;6:597–616. [PubMed] [Google Scholar]
- 24.Sikosek T., Chan H.S. Biophysics of protein evolution and evolutionary protein biophysics. J. R. Soc. Interf. 2014;11:1–35. doi: 10.1098/rsif.2014.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamaki K. The apoptotic initiator caspase-8: its functional ubiquity and genetic diversity during animal evolution. Mol. Biol. Evol. 2014;31:3282–3301. doi: 10.1093/molbev/msu260. [DOI] [PubMed] [Google Scholar]
- 26.Pop C., Chen Y.-R., Smith B., Bose K., Bobay B., Tripathy A., et al. Removal of the pro-domain does not affect the conformation of the procaspase-3 dimer. Biochemistry. 2001;40:14224–14235. doi: 10.1021/bi011037e. [DOI] [PubMed] [Google Scholar]
- 27.Ma C., MacKenzie S.H., Clark A.C. Redesigning the procaspase-8 dimer interface for improved dimerization. Protein Sci. 2014;23:442–453. doi: 10.1002/pro.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pop C., Oberst A., Drag M., van Raam B.J., Riedl S.J., Green D.R., et al. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem. J. 2011;433:447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackenzie S.H., Schipper J.L., England E.J., Thomas M.E., Blackburn K., Swartz P., et al. Lengthening the intersubunit linker of procaspase 3 leads to constitutive activation. Biochemistry. 2013;52:6219–6231. doi: 10.1021/bi400793s. [DOI] [PubMed] [Google Scholar]
- 30.Casey J.R., Grinstein S., Orlowski J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 31.Swietach P., Vaughan-Jones R.D., Harris A.L., Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:1–9. doi: 10.1098/rstb.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas M.E., Grinshpon R., Swartz P., Clark A.C. Modifications to a common phosphorylation network provide individualized control in caspases. J. Biol. Chem. 2018;293:5447–5461. doi: 10.1074/jbc.RA117.000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarado-Kristensson M., Melander F., Leandersson K., Rönnstrand L., Wernstedt C., Andersson T. p38-MAPK Signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J. Exp. Med. 2004;199:449. doi: 10.1084/jem.20031771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Wen W., Liu K., Zhu F., Malakhova M., Peng C., et al. Phosphorylation of caspase-7 by p21-activated protein kinase (PAK) 2 inhibits chemotherapeutic drug-induced apoptosis of breast cancer cell lines. J. Biol. Chem. 2011;286:22291–22299. doi: 10.1074/jbc.M111.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Q., Snipas S., Ortht K., Muzio M., Dixit V.M., Salvesen G.S. Target protease specificity of the viral serpin CrmA: analysis of five caspases. J. Biol. Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 36.Bhuyan A.K., Udgaonkar J.B. Folding of horse cytochrome c in the reduced state. J. Mol. Biol. 2001;312:1135–1160. doi: 10.1006/jmbi.2001.4993. [DOI] [PubMed] [Google Scholar]
- 37.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon J.C., Myers J.B., Folta T., Shoja V., Heath L.S., Onufriev A. H++: a server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005;33:W368–W371. doi: 10.1093/nar/gki464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanwell M.D., Curtis D.E., Lonie D.C., Vandermeerschd T., Zurek E., Hutchison G.R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012;4:1–17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith L.J., Berendsen H.J.C., van Gunsteren W.F. Computer simulation of urea-water mixtures: a test of force field parameters for use in biomolecular simulation. J. Phys. Chem. 2004;108:1065–1071. [Google Scholar]
- 41.Stumpe M.C., Grubmu H. Aqueous urea solutions: structure, energetics, and urea aggregation. J. Phys. Chem. B. 2007;111:6220–6228. doi: 10.1021/jp066474n. [DOI] [PubMed] [Google Scholar]
- 42.Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., et al. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. [Google Scholar]
- 43.Yu J.W., Jeffrey P.D., Shi Y. Mechanism of procaspase-8 activation by c-FLIPL. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8169–8174. doi: 10.1073/pnas.0812453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocco A.G., Mollica L., Ricchiuto P., Baptista A.M., Gianazza E., Eberini I. Characterization of the protein unfolding processes induced by urea and temperature. Biophys. J. 2008;94:2241–2251. doi: 10.1529/biophysj.107.115535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pop C., Feeney B., Tripathy A., Clark A.C. Mutations in the procaspase-3 dimer interface affect the activity of the zymogen. Biochemistry. 2003;42:12311–12320. doi: 10.1021/bi034999p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giebel R., Worden C., Rust S.M., Kleinheinz G.T., Robbins M., Sandrin T.R. Microbial fingerprinting using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS): applications and challenges. Adv. Appl. Microbiol. 2010;71:149–184. doi: 10.1016/S0065-2164(10)71006-6. [DOI] [PubMed] [Google Scholar]
- 47.Chalkley R.J., Hansen K.C., Baldwin M.A. Bioinformatic methods to exploit mass spectrometric data for proteomic applications. Methods Enzymol. 2005;402:289–312. doi: 10.1016/S0076-6879(05)02009-4. [DOI] [PubMed] [Google Scholar]
- 48.Macur K., Hagen L., Ciesielski T.M., Konieczna L., Skokowski J., Jenssen B.M., et al. A targeted mass spectrometry immunoassay to quantify osteopontin in fresh-frozen breast tumors and adjacent normal breast tissues. J. Proteomics. 2019;208 doi: 10.1016/j.jprot.2019.103469. [DOI] [PubMed] [Google Scholar]
- 49.Grinshpon R.D., Williford A., Titus-Mcquillan J., Clark A.C. The CaspBase: a curated database for evolutionary biochemical studies of caspase functional divergence and ancestral sequence inference. Protein Sci. 2018;27:1857–1870. doi: 10.1002/pro.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landau M., Mayrose I., Rosenberg Y., Glaser F., Martz E., Pupko T., et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005;33:W299–W302. doi: 10.1093/nar/gki370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waterhouse A.M., Procter J.B., Martin D.M.A., Clamp M., Barton G.J. Sequence analysis Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained in the article and supporting information.