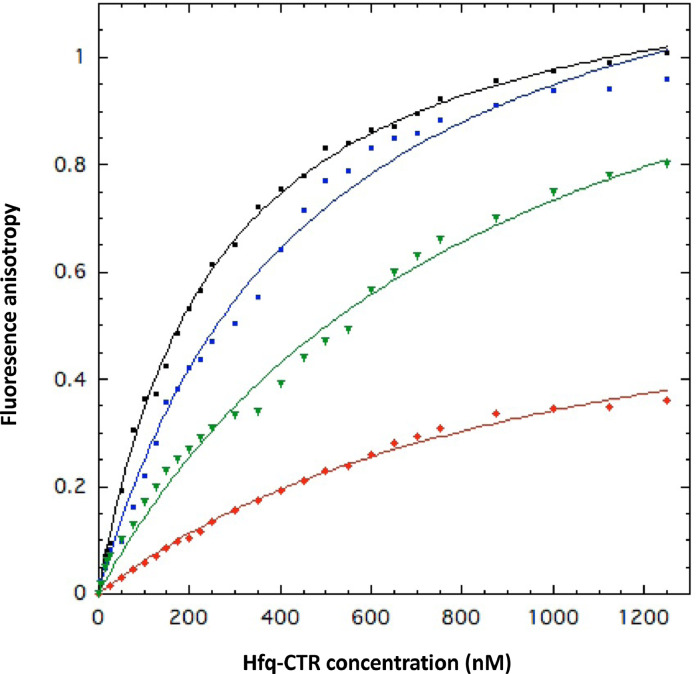
Fig. 2.
KD measurements of WT and mutated Hfq-CTR:dsDNA complexes using fluorescence anisotropy. In this case a dA:dT20 dsDNA was used. WT Hfq-CTR (black) has an equilibrium dissociation constant KD = 260 ± 10 nM; mutant Hfq-CTR S87A,S88A (blue) has a KD = 460 ± 30 nM. The mutants Hfq-CTR Y83A (red) and Hfq-CTR E99A,E100A,E102A (green) have lower affinities with KD = 1000 ± 75 nM and 890 ± 80 nM, respectively. For other mutants, namely Hfq-CTR S80A,S81A; Hfq-CTR R66A; Hfq-CTR S65A,S69A,S72A; Hfq-CTR S93A,S98A; Hfq-CTR H70A,H71A,H84A,H85A and Hfq-CTR G76A, G77A, G78A no complex is formed, titration curve was flat and not shown, in agreement with EMSA result.