Abstract
There have been numerous concerns about the disease and how it affects the human body since the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic began in December 2019. The impact of SARS-CoV-2 on the liver is being carefully investigated due to an increase in individuals with hepatitis and other liver illnesses, such as alcoholic liver disease. Additionally, the liver is involved in the metabolism of numerous drugs used to treat comorbidities and coronavirus disease 2019 (COVID-19). Determining how SARS-CoV-2 affects the liver and what factors place individuals with COVID-19 at a higher risk of developing liver problems are the two main objectives of this study. This evaluation of the literature included research from three major scientific databases. To provide an update on the current impact of COVID-19 on the liver, data was collected and relevant information was incorporated into the review. With more knowledge about the effect of the disease on the liver, better management and therapeutics can be developed, and education can ultimately save lives and reduce the long-term impact of the pandemic on our population.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Liver, Hepatic complications
Core Tip: We investigated the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on the liver due to an increase in individuals with hepatitis and other liver illnesses, such as alcoholic liver disease. Additionally, the liver is involved in the metabolism of numerous drugs used to treat comorbidities and coronavirus disease 2019 (COVID-19). Determining how SARS-CoV-2 affects the liver and what factors place individuals with COVID-19 at higher risk of developing hepatic issues are the two main objectives of this study.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly infectious pathogenic coronavirus that appeared in late 2019 causing a pandemic of acute respiratory disease, which is known today as coronavirus disease 2019 (COVID-19)[1]. At a fast rate, the virus spread worldwide, replicated, and mutated into multiple major variants, posing a threat to global public health. SARS-CoV-2 is part of the order Nidovirales, family Coronaviridae, subfamily Orthocoronavirinae, Betacoronavirus genus, and Sarbecovirus subgenus[1,2]. It is a single-stranded, positive-sense, enveloped ribonucleic acid (RNA) virus that is 79.6% identical to SARS-CoV-2 and 96.2% like a bat-derived coronavirus strain[2]. The host receptor for SARS-CoV-2 cell entry is identical to SARS-CoV-2, the angiotensin-converting enzyme 2 (ACE-2)[3]. SARS-CoV-2 binds to ACE-2 with a higher affinity to the receptor-binding domain (RBD) of its spike protein[3]. Therefore, SARS-CoV-2 is more infectious. Since the first reports, which were discovered in Wuhan, China's Hubei Province, at the end of 2019, cases have been documented on every continent[3]. Globally, more than 500 million confirmed cases of COVID-19 from exposure to SARS-CoV-2 have been reported[3]. SARS-CoV-2 tends to replicate in the upper and lower respiratory tract and is transmitted by droplets and aerosols from asymptomatic and symptomatic infected subjects[4]. Most infections occur between 2-14 d (about 2 wk) with an incubation period of 5-7 d[4]. These infections tend to be uncomplicated. A small percentage of patients are hospitalized due to severe inflammation and pneumonia. Complications tend to be respiratory and multiorgan failure[4]. Risk factors for complicated diseases are older age, diabetes, hypertension, chronic cardiovascular disease, chronic pulmonary disease, and immunodeficiency[4]. The distribution of COVID-19 cases across most countries is highest in the age group of 20-59 years old[4]. Major reductions in social interactions have been implemented in many countries with SARS-CoV-2 outbreaks, leading to rapid reductions. An estimate of the infection fatality rate that is currently reported is 0.5%-1.0%[4]. Despite a rapid worldwide spread, attack rates have been lowered in most regions, demonstrating the efficacy of control measures[4].
Based on initial COVID-19 data, both healthy individuals and those with pre-existing liver disease infected with the SARS-CoV-2 virus exhibit abnormal liver function tests (LFTs), implying that the virus may play a direct role in liver damage[5]. The incidence of liver injury in patients with COVID-19 has been estimated to range from 14.8% to 53.0%[6]. A clinical study showed that patients with stable liver cirrhosis who contracted the SARS-CoV-2 virus can experience rapid deterioration as evidenced by an increase in the Child-Pugh score[6]. The incidence of liver injury in cases of death from COVID-19 is 58.0%[6]. Liver injury following the contraction of the SARS-CoV-2 virus is characterized by hypoalbuminemia, hyperbilirubinemia, and an increase in alanine transaminase (ALT) and aspartate transaminase (AST)[5,6]. There may also be an increase in gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP), indicating injury to liver bile duct cells[6]. The degree of liver injury has a positive correlation with the severity of the infection. Mortality is statistically correlated with elevated AST and low albumin levels of 26.3-30.9 g/L[6]. The mechanism by which SARS-CoV-2 damages hepatocytes is still unclear; however, pathogenic mechanisms may include direct damage, immune-mediated, ischemia and hypoxia, thrombosis, and drug-induced[5,6]. This article aims to investigate the effects of SARS-CoV-2 on the liver and the risk factors for liver problems in coronavirus-infected patients.
METHODOLOGY
PubMed, Google Scholar, and Med Line Plus were used to conduct an electronic literature review. For the data compiled, the search was limited to peer-reviewed articles published between January 1, 2015, and July 1, 2022. The articles were chosen based on keywords such as coronavirus, COVID-19, SARS-CoV-2, and the effects of the virus on the liver. The articles were then examined and included depending on the topic's applicability.
REPORTED SYMPTOMS OF SARS-CoV-2 LIVER INJURY
Elevated LFTs in COVID-19
Altered LFTs have been observed in almost half of the hospitalized patients with COVID-19 infection[7]. In particular, elevated levels of liver enzymes glutamic-pyruvic transaminase (ALT), glutamic-oxaloacetic transaminase (AST), glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), and bilirubin have been seen to manifest as liver injury in such patients[6,8]. Previous studies have shown that the incidence of COVID-19 liver damage with elevated ALT ranges between 9.6% and 37.6%, elevated AST between 14.8 and 36.0%, and the proportion of abnormal GGT between 13.0%-24.4%[9]. These abnormal tests are the result of increased AST and ALT, whereas AST was more common than ALT. In addition, 10.5% to 69.0% of hospitalized COVID-19 patients showed abnormal LFTs. Hypoalbuminemia has also been reported as a consequence of COVID-19-related liver injury and was observed more significantly in men with COVID-19 compared to women[10]. When comparing and analyzing those with severe and non-severe COVID-19 cases, liver function abnormalities like hypoalbuminemia, GGT, aminotransferase, and bilirubin elevations were more frequent in those with severe disease as opposed to mild/moderate forms of the infection[11]. The liver injury caused by COVID-19 was related to the degree of severity of the infection and manifested as different degrees of liver function abnormalities[12].
Pathological changes in liver biopsies
Histopathological findings from liver biopsies of COVID-19 patients showed moderate microvascular steatosis with lobular and portal vein involvement[13]. Hepatocyte degeneration, with neutrophil infiltration of the hepatic lobes and sinusoidal enlargement of the central lobule, was observed. Congestion of hepatic sinuses with micro thrombosis and sinusoidal expansion, lymphocytic infiltration of the lobes, and hepatic necrosis in the periportal and centrilobular segments was also identified in patients[14]. Furthermore, pathological findings showed hepatocellular necrosis, cellular infiltration, an increase in the number of mitotic hepatocytes, and fatty degeneration[15]. In addition, COVID-19 Liver injury showed an elevation of eosinophilic bodies along with dilated hepatocytes[6]. Sinusothelial micro thrombosis disease was evident in approximately 20.0% of cases with focal endothelial damage[16]. Acinar atrophy was depicted in autopsy specimens in the late course of the infection[17]. Increased liver stiffness is also correlated with increased levels of biomarkers of liver injury, such as ALT and GGT, suggesting underlying hepatocellular and cholangiocellular damage at the biochemical level[18]. Changes in liver elasticity, viscosity, and steatosis levels were also observed in liver tissues in COVID-19 patients, with increased fibrosis compared to the control group (P < 0.001)[18]. In some studies, the liver appeared pale and yellowish on sectioning, with a nutmeg appearance[19]. The infection has been studied to cause cholangiocellular injury and cholestasis and consequently bile duct proliferation, with bile plug formation. In general, analyses have revealed that as the severity of COVID-19 in a patient increase, the levels of AST, ALT, total bilirubin, GGT, and ALP increase, resulting in a greater degree of liver injury, as observed in hepatocytes[20].
SARS-CoV-2 RELATED LIVER INJURY: PATHOGENIC MECHANISMS
Liver injury in patients infected with SARS-CoV-2 occurs via several mechanisms[21]. Several studies indicate that liver injury in patients with SARS has manifested through the elevation of liver enzymes, mainly ALT and/or AST in the early stage of the infection[21]. The incidence of liver injury in SARS patients ranges from 14.8% to 53.0%[21]. One hypothesis for the cause of liver injury is a direct invasion of the hepatic parenchyma by SARS-CoV-2[21]. Autopsy of patients with SARS found a large number of virus particles in the parenchyma and vascular endothelium of the liver[21]. The main receptor used by SARS to enter cells is ACE-2 which is abundantly present in cholangiocytes, endothelial cells, and the progenitor cells of the liver[22]. This results in acute liver and hepatitis biopsies in postmortem patients showing a significant increase in macrovesicular steatosis with eosinophilic bodies and high levels of mitotic cells suggesting hepatocyte apoptosis[21]. The results suggest that SARS infection causes direct injury to the hepatic parenchyma and concomitantly compromises the regenerative capability of the liver[22]. Hepatic injury is further exacerbated by the body’s immune response to severe COVID-19 infection[23]. SARS activates both the innate and acquired immune system resulting in the release of high levels of several inflammatory cytokines by immune cells[23]. The resulting cytokine storm in severe SARS infections is the cause of death in 28.0% of fatal cases of COVID[24]. Multiorgan failure is a sequela of the cytokine storm, and the liver is no exception. Critically ill patients exhibited increased levels of interferon-lambda (IFN- λ), transforming growth factor-alpha (TGF-α), thymic stromal lymphopoietin, interleukin-16 (IL-16), IL-23, IL-33, and markers linked to coagulopathy, such as thrombopoietin. Patients with severe COVID are commonly anoxic due to respiratory failure. This requires patients to be mechanically ventilated and/or on vasopressor support. Lower cardiac output has a detrimental impact on the hemodynamics of the liver[23]. The resultant reduced hepatic blood flow can lead to anoxic hypoxic hepatitis and/or cholestasis[24]. Another complication of hepatic injury due to the high levels of inflammatory cytokines released by the body includes thrombosis and vascular congestion of the liver, which have been observed in autopsy samples of patients with severe COVID-19. Patients with severe COVID-19 were found to have elevated levels of total bilirubin and ALT, as well as elevated levels of inflammatory biomarkers such as IL-6, IL-10, C-reactive protein (CRP), and D-dimer. One of the mechanisms contributing to the damage observed in the liver of these patients is due to the SARS-CoV-2 infected cells which upregulate and produce large amounts of cytokines to help combat the virus, resulting in collateral damage to both infected and uninfected cells. This hyper-stimulated systemic inflammatory response induces macro- and micro-circulatory dysfunction, leading to global hypo-perfusion resulting in hypoxia, hypo-tension, and a hypercoagulable state. Therefore, microvascular thrombosis should be considered an important cause of liver injury and dysfunction in patients with COVID-19[25]. Hepatic injury during COVID-19 infection can be exacerbated by medications leading to elevated levels of ALT and AST. Remdesivir has shown in vitro antiviral activity against SARS-CoV-2 and a shorter recovery time in clinical trials, but elevated hepatic enzymes have also been reported as a major adverse drug reaction. Although some studies attribute this abnormal increase to viral infection rather than the side effect of the drug, others proposed that, whether or not it was affected by SARS-CoV-2, remdesivir increased the risk of hepatotoxicity. Other commonly used drugs to treat COVID-19 such as lopinavir/ritonavir have also resulted in hepatic injury. ACE medications and angiotensin II receptor blockers, which take a more focused approach, have also been reported to raise liver enzymes in COVID-19 patients. In autopsies of COVID-19 deaths, moderate microvesicular steatosis and mild lobular and portal activity were observed and probably associated with drug-induced liver injury. Other medications used for patients with COVID-19 that can trigger liver injury include antibiotics such as macrolides and quinolones, antivirals such as ribavirin, and even steroids[26].
SARS-CoV-2 INFECTION AND PRE-EXISTING LIVER DISEASES
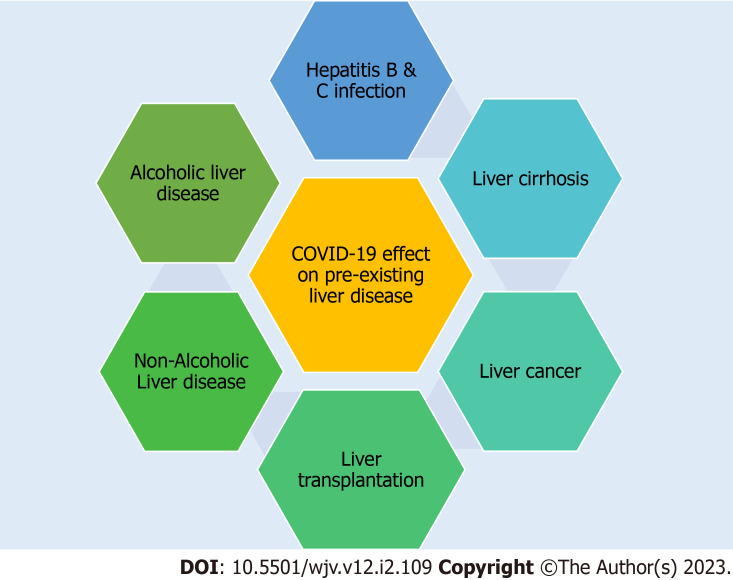
The effect of COVID-19 on pre-existing liver diseases discussed below are hepatitis B and C virus infection, liver cirrhosis, liver cancer, liver transplant, non-alcoholic fatty liver disease (NAFLD), and alcoholic liver disease (Figure 1).
Figure 1.
Effect of coronavirus disease 2019 and preexisting liver disease.
COVID-19 effect on hepatitis B virus and hepatitis C virus infection
Hepatitis C virus (HCV) and hepatitis B virus (HBV) were reported as the leading causes of liver diseases, but no specific data on the prevalence of these infections were provided. Many studies involving patients with COVID-19 showed a relatively low prevalence of chronic liver disease (CLD) at baseline, equal to 3.0%. Similarly, a prevalence rate of 3.0% CLD is associated with documented underlying chronic HBV or HCV infections in specific populations[27]. In one study of patients admitted to hospitals for COVID-19 in the northeastern United States, HCV infection was observed in P < 0.1% of patients, but information on HCV RNA levels was insufficient. In the same study, 23 cases (3.8%) presented positive HCV serology, of whom six patients (0.99%) had detectable viral HCV load at the time of hospital admission for the diagnosis of COVID-19[27]. In contrast, 2.0% of all patients showed hepatitis B surface antigen (HBsAg) positive chronic infection[27]. The study showed patients with recorded chronic HBV or HCV infection did not experience a more severe clinical course of COVID-19 compared to patients with HBsAg negative or undetectable HCV RNA, which measured the delay in SARS-CoV-2 clearance in HBV patients. Similarly, median viral clearance was not affected by preexisting HBV or HCV infection[27]. Lastly, chronic HBV or HCV infection (in the absence of cirrhosis) did not affect the prognosis of COVID-19 in the United States population.
COVID-19 effect on liver cirrhosis
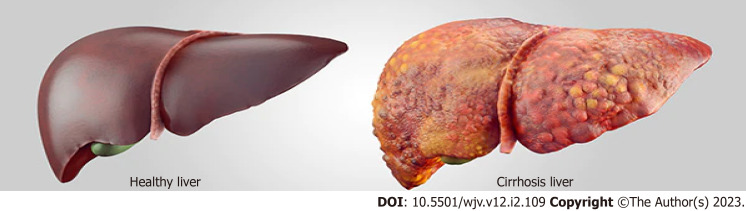
Liver cirrhosis (Figure 2) increases the mortality of SARS-CoV-2 viral infection[28]. The path-ophysiological mechanism for the SARS-CoV-2 virus begins with the spike glycoprotein (S) to allow viral entry into the target cell. The virus replicates to infect other surrounding cells through the ACE-2 receptor in cholangiocytes and hepatocytes to cause biliary and liver symptoms. Elevated liver enzymes are multifactorial and strongly associated with liver injury. A prevalent hepatic phenomenon associated with SARS-CoV-2 infection presents with elevated ALT and AST levels, with abnormal ALP and bilirubin readings[29]. Elevated serum levels of GGT, a marker of hepatobiliary cell injury, are found in a quarter of patients hospitalized with COVID-19[28]. Higher levels of a hepatocellular enzyme associated with severe cases of COVID-19 directly affect mechanisms that include increased cytokine release in the viral presence or microthrombotic ischemic liver injury[29]. Although respiratory symptoms are the most reported among COVID-19 patients, these pulmonary manifestations are vulnerable to decompensated liver cirrhosis but are currently understudied. A cohort study with 250 patients with prior CLDs reported high mortality in patients with cirrhosis (RR: 4.6, 95.0%CI: 2.6-8.3)[28]. In an international study, a positive correlation was shown that patients with cirrhosis are predisposed to significant toxic liver injury due to SARS-CoV-2 infection, as acute-on-chronic liver failure had occurred in 20.0% of patients who experienced severe cirrhosis with COVID-19[28]. The viral ability of SARS-CoV-2 to bind to ACE-2 receptors on epithelial cells of the bile duct demonstrates its ability to affect liver regeneration capabilities and immune response[28]. By affecting the innate immunity of the reticuloendothelial system, the immunosuppressed state causes a cytokines-mediated reaction, resulting in liver decompression[28].
Figure 2.
Healthy and cirrhosis liver.
COVID-19 effect on liver cancer
Pre-existing liver diseases are considered risk factors for poorer prognosis in COVID-19 as various pathophysiological processes result in liver damage due to SARS-CoV-2 infection. Biochemical presentations of liver injury include elevated levels of ALT/AST, ALP, GGT, or total bilirubin above the normal range. The decrease in lymphocyte count and the increase in neutrophil counts demonstrate the role of innate immunity in COVID-19-associated hepatic injury. Postmortem studies in liver histology in COVID-19 patients show moderate microvascular and macrovascular steatosis with mild inflammation of the lobular portal. This highlights the pathological changes observed in hepatocellular carcinoma (HCC). Elevated levels of eosinophils are observed in autopsy studies in centrilobular steatosis, in addition to the increased number of mitotic cells[30]. Patients with HCC are closely monitored, as increased inflammation due to COVID-19 may predispose patients to post-hepatectomy liver failure. Furthermore, COVID-19 can potentially exacerbate CLD and alter treatments for cancer patients with a higher risk of infection and poor outcomes[30]. The management and monitoring of patients with HCC are performed by imaging (magnetic resonance imaging, ultrasound) and measuring alpha-fetoprotein levels[29]. The practice guidelines recommend the establishment of surveillance intervals to reduce the radiologic capacity of at-risk patients, with a 98.0% estimate that at-risk patients would not develop HCC during each surveillance interval. Locoregional and systematic therapies are recommended for advanced HCC treatment. However, oral therapy with tyrosine kinase inhibitors (i.e., sorafenib and lenvatinib) and immunotherapy effectively serve as first-line therapies to reduce exposure[29].
COVID-19 effect on liver transplantation
With the severity of SARS-CoV-2 infection dependent on comorbidities (i.e., cardiovascular disease and diabetes mellitus), underlying liver diseases do not necessarily influence the outcome of COVID-19 infection. Solid-organ transplants, including liver transplant (LT) recipients, are increasingly susceptible to severe infections due to chronic immunosuppression, thereby increasing the risk for severe COVID-19 infection. A meta-analysis that included 17 articles and the outcomes of 1481 COVID-19 LT patients was compared with 239704 non-LT patients infected with COVID-19. From 17 articles, a cumulative incidence of mortality of 17.4% (95.0%CI, 15.4-19.6) was found among LT recipients with COVID-19 with causes of death reported as 62.54% by COVID-19-related complications (95.0%CI, 56.24-68.55), 29.88% by pulmonary failure (95.0%CI, 24.28-36), and 1.6% liver-related (95.0%CI, 0.1-2.84). Mortality was proportionate between LT and non-LT patients [OR, 0.8 (0.6-1.08); P = 0.14][31]. Twelve studies in the same meta-analysis reported that 23.0% of LT patients who had developed a severe COVID-19 infection were positive with symptoms that included fever (49.7%), cough (43.76%), dyspnea (29.27%), and symptoms gastrointestinal (27.26%)[31]. Eight studies from the same meta-analysis reported modification change immunosuppression in 55.9% of LT recipients infected with COVID-19[31]. Comorbidities such as hypertension, diabetes, and obesity were common in infected patients where 72.0% of the patients were hospitalized, and 16.0% required care in the intensive care unit (ICU)[31]. Although hospitalization of LT recipients far exceeded non-LT patients [OR, 1.99 (1.41-2.8), P < 0.001], the ICU care requirement was comparable between groups, as the cumulative incidence of graft dysfunction was 2.3% (1.3-4.1)[31].
COVID-19 effect on non-alcoholic fatty liver disease
Many observational studies have shown that patients with comorbidities such as cardiovascular disease, arterial hypertension, diabetes mellitus, CLD, or cancer are susceptible to more severe episodes of COVID-19, as seen in NAFLD and other less common disorders[32]. Recently, several meta-analyses have shown that obesity and diabetes (both strongly associated with NAFLD) are significantly associated with the progression of more severe disease and increased mortality in patients with COVID-19[32]. They have reported a six-fold increased risk of severe COVID-19 in the presence of obesity in NAFLD. In addition, a meta-analysis showed that obesity could exacerbate COVID-19 infection. Patients with severe COVID-19 disease had higher body mass indices, and obesity was associated with the development of the disease, the need for care, and admission to an ICU[32]. The risk of severe COVID-19 in obese patients was more significant than in younger patients. This suggests that NAFLD patients are at increased risk of liver damage, although liver enzyme levels at admission or during hospitalization were generally not significantly elevated. Furthermore, NAFLD was not associated with adverse clinical outcomes in younger patients with COVID-19. Another study found that NAFLD was more common in patients with severe COVID-19 than in stable patients. However, the mean age and the number of comorbidities were also significantly higher in patients with severe COVID-19 infections[32]. Patients with NAFLD had a substantially higher risk of disease progression, more likely changes in liver enzymes, and longer viral shedding times than non-NAFLD patients. Non-NAFLD patients showed that moderate to high Fibrous-4 and NAFLD fibrosis scores were strongly and independently correlated with the severe progression of COVID-19 disease[32].
COVID-19 effect on alcoholic liver disease
The ACE-2 receptor is exceedingly expressed in alveolar type II cells and liver and bile duct cells, making it significantly feasible for SARS-CoV-2 to infect cells in those areas. Especially, cholangiocytes have a specific ACE-2 receptor in more concentrations than hepatocytes, making them more susceptible to COVID-19 infection. However, because the liver harbors a widespread quantity of macrophages, generating an ample cytokine-mediated immune reaction, hepatocytes can also be prone to a SARS-CoV-2 infection. Patients with COVID-19 patients with liver cirrhosis have always shown elevated levels of ALT, AST, D-dimer, CRP, IL-6, and ferritin. Although the current literature is limited, research has proven that people with CLDs could have increased models for end-stage liver disease and undergo extended liver and pulmonary complications while infected with COVID-19. Specifically, the mortality rate in patients with preexisting liver disease is 1.8%. Lastly, the severity of liver harm due to COVID-19 infection tends to be substantially worse and more widespread in people with pre-existing alcoholic liver cirrhosis than in those without[33].
ANTI-SARS-CoV-2 TREATMENTS EFFECTS ON THE LIVER
Currently, various treatments for COVID-19 (SAR-CoV-2) are being investigated, some of which may be associated with hepatotoxicity[34]. Remdesivir (RDV), an antiviral medication, was initially developed and tested for the treatment of hepatitis C and later the Ebola and Marburg viruses. Amid the COVID-19 pandemic, RDV was approved for emergency use to treat COVID-19 in many countries[35]. In patients diagnosed with COVID-19, in vitro and in vivo studies indicate that RDV has an antiviral effect on SARS-CoV-2[36]. Various medication-related adverse events include but are not limited to reasonable degrees of nausea and vomiting, headache, fatigue, renal dysfunction, and rash[37]. The risk of an adverse event involving the liver exists as one of the clearest potential risks from RDV[38]. RDV therapy is administered intravenously for 3 to 10 d and is often accompanied by reversible mild to moderate elevations in serum AST levels, but has been rarely associated with clinically apparent liver injury. Effects on the liver range from asymptomatic to mild-with elevations in serum ALT and AST upon introduction of RDV therapy in patients with COVID-19. The systemic effects of COVID-19 likely overshadow the outcomes of hepatic involvement[37]. However, there is uncertainty regarding if the effect on liver enzymes is due to remdesivir, COVID-19 solely, or both[39,40]. It is recommended that patients remain under the supervision of health professionals to monitor liver health before and during remdesivir infusions[37]. To fully assess the risk of remdesivir-associated liver damage, more studies are necessary for this area[40]. Lopinavir (LPV) is an antiretroviral protease inhibitor, used together with ritonavir (booster) in the prevention and treatment of human immunodeficiency virus (HIV) infection[41]. Lopinavir/ritonavir (LPV/r) was developed to inhibit HIV protease, the primary distinction for the SARS-CoV-2 counterpart (3CLpro) lies within the varying spatial structure of the HIV aspartic protease as compared with 3CLpro cysteine protease[41]. The LPV/r combination improves LPV pharmacokinetics by decreasing liver metabolism by inhibiting the cytochrome (CYP) P450 3A4 enzyme[41].
A randomized controlled study in adult patients hospitalized with COVID-19 shows that of those adults treated with LPV/r, only one individual in the LPV/r group presented elevated ALT, more than 2.5 times above the normal limit[41,42]. When comparing patients treated with LPV/r to patients in the control group, there was no evidence of liver dysfunction noted in controls. It is important to acknowledge that the patient presenting with an elevated ALT had a pre-existing chronic liver condition, possibly contributing to the liver disturbance[42]. Another research study suggests that no observable side effects were found in the LPV/r group, except for transient elevation of ALT elevation (< 125 U/L) in three patients[43]. Given that none of the patients progressed to a severe clinical status at the end of the follow-up period, it is believed that LPV/r treatment rarely causes harm in patients recovering from COVID-19[42]. LPV/r is considered an independent factor for liver injury[44]. Interferons (IFNs) are natural antiviral immune modulators that help the body’s immune system defend against infection and disease, including viruses and cancer[45]. Studies have shown that during SARS and middle east respiratory syndrome, Type I IFNs are markedly suppressed and the administration of exogenous Type I IFNs has been shown to reduce the severity of the symptoms of these diseases[46]. To assess the effectiveness and safety of interferon β-1a (IFN β-1a) in patients with severe COVID-19, a randomized clinical trial was conducted[47]. Comparisons were made between patients receiving IFN and those receiving controlled standard therapy. Hepatic complication rates were measured between patients in the treatment group and those patients receiving standard care while the preexisting liver disease was considered[44]. The frequency of hepatic failure did not differ between the IFN and control groups (11.90% vs 23.07%), suggesting that IFN-β-1a may not be a major factor in the liver damage seen by COVID-19 patients[47]. Studies show that IFNs used to treat patients with COVID-19 are unlikely to be associated with liver disease. IFNs may lead to hepatic toxicity when combined with other drugs[44]. To authenticate these results, additional studies are necessary[44]. Baricitinib is a JAK-STAT inhibitor used to treat individuals with rheumatoid arthritis who cannot tolerate more than one tumor necrosis factor (TNF) antagonist[6]. By decreasing adaptor-associated kinase 1 activity, a regulator of clathrin-mediated endocytosis, baricitinib has been shown to affect the hyperinflammatory state that developed during SARS-CoV-2 infection and may prevent endocytosis and viral infection[6,48]. Furthermore, the oral administration of baricitinib and the excellent pharmacokinetic profile (very short half-life, low plasma protein binding, and minimal interference with CYP enzymes) make it a viable combination therapy with direct-acting antivirals such as LPV/r and RDV[48]. The growing number of reports of infections and thrombosis following the use of JAK inhibitors for the treatment of COVID-19 should be taken seriously as liver damage, cholestasis, and hepatitis unexpectedly manifested in a non-negligible fraction of individuals[6]. Furthermore, these unfavorable hepatic consequences should be evaluated. Tocilizumab is a monoclonal antibody that is used to block the inflammatory protein IL-6. Tocilizumab improves joint pain and swelling from arthritis and reduces other symptoms caused by inflammation. More recently, tocilizumab use has been indicated for the treatment of cytokine release syndrome in patients with COVID-19 infection[49]. Common side effects of tocilizumab include a runny or stuffy nose, sinus pain or sore throat, headache, or dizziness. The most common side effects of tocilizumab include headache and hypertension but, rarely, hepatotoxicity ranging from mild transaminase elevation to severe drug-induced liver injury can occur[50].
SARS-CoV-2 VACCINES EFFECT ON THE LIVER
It is not yet clear whether RNA or DNA-based vaccines have any direct effect on the liver, resulting in hepatotoxicity. Although anti-coronavirus treatments have been found to cause mitochondrial and endoplasmic reticulum dysfunction, the effects of vaccines require further testing[6]. Patients receive what are often considered benign mRNA vaccines for Crigler-Najjar syndrome and rabies, which have some form of hepatotoxicity. While there is no definitive cause, there seems to be a potential link between the two. This is not the case with DNA vaccines, which makes them strikingly different. Immune system stimulation occurs via a completely different mechanism than mRNA vaccines, with IFN-1 secretions triggering the immune response. Unlike mRNA vaccines, DNA vaccines do not require subsequent doses to maintain monoclonal antibody protection, making DNA vaccines not only potentially more efficacious than mRNA vaccines, but requiring lower amounts to achieve a less toxic overall therapeutic effect. More research is needed to fully understand the mechanisms involved and how they affect the liver. Existing studies exclude patients with chronic liver disease as they are contraindicated by mRNA vaccines, making little information available regarding the pathophysiology, and comparing that of otherwise healthy individuals[6]. Patients with CLD are at an increased risk of infection, which is expected given the insufficient immune response. Vaccinations are imperative to reduce mortality in patients with CLD[51]. According to the Advisory Committee on Immunization Practices (ACIP), patients with CLD should be vaccinated against SARS-CoV-2 and influenza, pneumococcus, tetanus, diphtheria, pertussis, herpes zoster, hepatitis A, and hepatitis B[52]. Specifically, with the COVID-19 vaccine, ACIP suggests an mRNA vaccine with a booster dose five months after completing the two scheduled doses. For severely immunosuppressed patients, a booster dose is recommended after three months to strengthen the immune response[53]. A double-blind randomized trial studying the administration of a third dose of the mRNA vaccine in transplant recipients showed a strong immune response compared to the placebo group[54]. In a case report of healthy patients with no history of liver disease, patients developed jaundice and elevated liver enzymes after administration of the mRNA vaccine, either after the first or second dose. In these cases, the laboratory values and symptoms resolved without treatment after several weeks. The data suggests that this response is due to a neutrophil-predominant inflammatory response[55]. Comparatively, much data shows contraindications to multiple COVID-19 vaccines, including booster doses, specific to CLD patients. Data are still needed to assess the efficacy and long-term effects of multiple COVID-19 vaccines in patients with CLD.
The severity of the liver disease may be assessed using the Child-Pugh scale. This scale anticipates mortality in CLD and is categorized into three stages: good hepatic function, moderately impaired hepatic function, and advanced hepatic dysfunction[56]. Multiple factors, including the stage of CLD, can be a determinant of the efficacy of a vaccine, and those in later stages are more susceptible to infections and adverse events. This is possibly due to the inefficiency of the body in producing an adequate immune response[51]. However, the World Health Organization (WHO) currently recommends that COVID-19 vaccines for those with deficient immune systems be given additional boosters to help increase a sufficient immune response[57]. It is recommended that vaccinations be administered as early in the disease process as possible to gain the best performance of the vaccine[52]. In a study on the antibody response of the vaccine in CLD patients, subjects were given the recommended series of the mRNA vaccine. The results showed that 24.0% of the subjects had a poor antibody response[58]. Additional research is needed to further assess the success of the vaccine in varying severities of CLD.
DISCUSSION
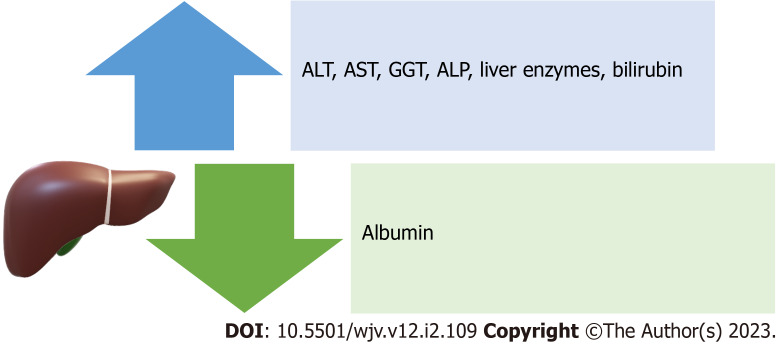
Lessons from previous coronavirus outbreaks and other viral epidemics indicate that the combination of systemic and partial inflammatory responses induced by these infections may result in severe respiratory syndromes and related complications (such as abnormal liver function, cardiac insufficiency, and renal failure)[6]. Manifestations of liver damage from SARS-CoV-2 include a decrease in albumin and an increase in ALT, AST, liver enzymes, and bilirubin[9]. Increases in GGT and ALP are also seen in COVID-19 patients, indicating liver damage to bile duct cells[7] (Figure 3). Since liver biopsies taken from a small number of COVID-19 patients did not reveal viral inclusions, but rather a macro-vesicular steatosis, liver damage may also be the result of bile duct cell damage[59]. The pathogenic alterations frequently take the form of macro-vesicular steatosis and mild lobular and portal inflammation[59,60]. According to earlier research, severe instances of coronavirus infection had a strikingly higher frequency and severity of liver impairment than moderate cases. The mechanism by which coronaviruses harm hepatocytes and influence hepatic function is still unknown, even though multiple clinical studies have shown a high link between coronaviruses and liver damage[6]. Potential mechanisms of liver injury that have been reported include immune-mediated damage because of the severe dysregulated inflammatory response, direct cytotoxicity, systemic hypoxia with hypoxic hepatitis, drug-induced liver injury, reactivation of pre-existing liver disease, mitochondrial dysfunction, SARS-CoV-2-induced hepatic steatosis, microthrombotic disease, ischemic hepatitis, cardiomyopathy with hepatic congestion, and extrahepatic release of transaminases[61]. Ischemic, hypercoagulable, and hyperinflammatory states are independent predictors of death in patients with COVID-19 and not liver injury[62]. Coronavirus infection significantly increases immunological activation. Numerous cytokines and chemokines (IL-6, IL8, IFN, and TNF, among others) are generated by immune cells after coronavirus infection and released into the blood, causing inflammation in different organs or even acute respiratory distress syndrome and multiple organ failure, suggesting that coronavirus-induced systemic inflammatory response syndrome (SIRS) and cytokine storms are important causes of liver damage[6,25]. This shows that immunotherapy is necessary for individuals with coronavirus infection, and as a result, corticosteroids and interferons are frequently utilized due to their ability to reduce inflammation[6]. Hypoxia may result in a long-term increase in reactive oxygen species, which may encourage the release of a variety of inflammatory mediators that harm the liver[6,25]. As a result, it will be important to keep an eye on patients' hypercoagulable conditions, such as thrombocytopenia and elevated levels of D-dimer and ALP, to prevent thrombosis and additional ischemia and hypoxia[6,23].
Figure 3.
Manifestations of liver damage from severe acute respiratory syndrome coronavirus 2. ALT: Alanine transaminase; AST: Aspartate transaminase; GGT: Gamma-glutamyl transferase; ALP: Alkaline phosphatase.
Immune compromise is typically caused by hepatitis B and C, liver cirrhosis, liver malignancy, and immunosuppressive medications after liver transplantation[6]. The severity and mortality rate in HBV infection patients are higher than in those with negative HBV due to delayed clearance of SARS-CoV-2[6]. The Child-Pugh scores of those who have already developed liver cirrhosis are likely to rise due to liver injury caused by COVID-19[56]. Furthermore, COVID-19 complications occur earlier and to a greater extent in patients with systemic immunocompromised status[6]. COVID-19 also has a significant impact on the treatment of liver diseases[11]. The discontinuation of high-dose corticosteroid therapy in hepatitis B and C patients receiving anti-HBO treatment may result in HBV reactivation during SARS-CoV-2 infection[6]. In addition, lopinavir and ritonavir have been shown to increase the risk of developing liver injury in HBV or HCV infection patients[6,11]. Coronavirus infection is currently treated with redelivering, lopinavir/ritonavir, interferon-a, baricitinib, and tocilizumab. The difficulty in developing optimized drugs for coronavirus infection is mainly due to severe side effects. Remdesivir, lopinavir, and ritonavir have all been linked to an increased risk of liver injury, with the severity of the injury being closely related to the dose of these drugs. IFNs have the potential to trigger a non-specific immune response, resulting in hepatocyte damage and autoimmune hepatitis, as well as an increased risk of developing severe complications such as systemic inflammatory reaction syndrome and acute respiratory distress syndrome[6]. Baricitinib, as a JAK inhibitor, can increase the risk of thrombosis and cause liver damage[6,48]. Tocilizumab can also reactivate HBV in SARS-CoV-2 co-infection, causing both viral hepatitis and COVID-19 recovery to be delayed; whereas other studies have shown hepatotoxicity as a potential side effect[50]. Overall, coronavirus vaccines will be critical in preventing outbreaks, but several factors must be considered to avoid an activated innate inflammatory response, an increase in the incidence of autoimmune diseases, and vaccine-induced liver injury[6].
Furthermore, a study observed 900 patients (32.2% in the 18-39 age group, 39.7% in the 40-69 age group, and 28.1% in the 70+ age group) with SARS-CoV-2[63]. It was seen that those with comorbidities, median D-dimer, and CRP levels all increased with age. AST/ALT and ALP/GGT levels also increased significantly during COVID-19[63]. Patients with elevated hepatocellular transaminases (AST/ALT) and cholestasis parameters (ALP/GGT/bilirubin) were found in 40.3% (n = 262/650) and 45.0% (n = 287/638), respectively[61]. Importantly, patients between the ages of 40 and 69 were more likely to experience COVID-19-associated liver injury (16.0%, P < 0.001), abnormal liver chemistry, and liver-related death (6.5%, P < 0.001)[61]. After the initial SARS-CoV-2 polymerase chain reaction result was positive, elevated AST and bilirubin levels independently predicted mortality in the entire population and patients aged 40 to 69 years[63].
CONCLUSION
The incidence of liver injury in patients with COVID-19 has been estimated to be as high as 53.0%. Those affected by COVID-19-associated liver injury generally fall between the ages of 40 and 69. The mechanism by which SARS-CoV-2 damages hepatocytes is still unclear. However, the SARS-CoV-2 virus may play a direct role in liver damage because both healthy individuals and those with preexisting liver disease exhibit abnormal LFTs. The liver injury caused by COVID-19 is related to the degree of severity of the infection and manifests itself with different degrees of liver abnormalities. The degree of liver injury manifested by AST, ALT, total bilirubin, GGT, and ALP has been shown to have a positive correlation with the severity of the disease. Interestingly, some studies have even shown that mortality correlates with elevated AST and low albumin levels. Furthermore, SIRS and cytokine storms augment liver injury and dysfunction. Commonly used medications may play a role in liver hepatotoxicity, however further studies are necessary. Pre-existing liver diseases are considered risk factors for worse prognosis in COVID-19, specifically, liver cirrhosis was shown to increase the mortality in these patients. Although vaccines have significantly changed the course of this pandemic, CLD is a contraindication of multiple COVID-19 vaccines. However, the increased severity of liver disease in determining the immune response to the COVID-19 vaccine is still unclear, and more studies are required in this area. As more information about the virus becomes available, it will be critical to comprehend the pandemic's effects on the liver, as well as the possible long-term consequences, especially in the immunocompromised population.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 6, 2022
First decision: December 19, 2022
Article in press: February 1, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Nigeria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beyoglu MA, Turkey; Zhou C, China S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
Contributor Information
Adekunle Sanyaolu, Department of Public Health, Federal Ministry of Health, Abuja, Nigeria, Abuja 0000, FCT, Nigeria. sanyakunle@hotmail.com.
Aleksandra Marinkovic, Department of Basic Medical Science, Saint James School of Medicine, The Quarter 2640 0000, Anguilla.
Abu Fahad Abbasi, Department of Internal Medicine, Loyola University Medical Center, Maywood, Illinois, IL 60153, United States.
Stephanie Prakash, Department of Basic Medical Science, Saint James School of Medicine, The Quarter 2640 0000, Anguilla.
Risha Patidar, Department of Basic Medical Science, Saint James School of Medicine, The Quarter 2640 0000, Anguilla.
Priyank Desai, Department of Basic Medical Science, American University of Saint Vincent School of Medicine, Saint Vincent and the Grenadines 0000, Saint Vincent and the Grenadines.
Martina Williams, Department of Basic Medical Science, Saint James School of Medicine, The Quarter 2640 0000, Anguilla.
Abdul Jan, Department of Basic Medical Science, Windsor University School of Medicine, Cayon 0000, Saint Kitts and Nevis.
Kareem Hamdy, Department of Basic Medical Science, Saint James School of Medicine, The Quarter 2640 0000, Anguilla.
Rachael Solomon, Department of Basic Medical Science, Caribbean Medical University School of Medicine, Willemstad 0000, Curaçao, Netherlands Antilles.
Vyshnavy Balendra, Department of Basic Medical Science, Saint James School of Medicine, The Quarter 2640 0000, Anguilla.
Maaz Ansari, Department of Basic Medical Science, Saint James School of Medicine, The Quarter 2640 0000, Anguilla.
Omar Shazley, Basic Medical Science, Saint James School of Medicine, Saint Vincent and the Grenadines 0000, Saint Vincent and the Grenadines.
Nasar Khan, Department of Basic Medical Science, Windsor University School of Medicine, Cayon 0000, Saint Kitts and Nevis.
Rochelle Annan, University of Health Sciences Antigua School of Medicine, Piccadilly, St. John's Antigua.
Yashika Dixon, Department of Basic Medical Science, Windsor University School of Medicine, Cayon 0000, Saint Kitts and Nevis.
Chuku Okorie, Department of Science, Union County College, Plainfield, New Jersey, NJ 07016, United States.
Afolabi Antonio, Department of Internal Medicine, Lloydminster Regional Hospital, Lloydminster S9V 1Y5, Saskatchewan, Canada.
References
- 1.Ravi V, Saxena S, Panda PS. Basic virology of SARS-CoV 2. Indian J Med Microbiol. 2022;40:182–186. doi: 10.1016/j.ijmmb.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Li JZ. SARS-CoV-2 Virology. Infect Dis Clin North Am. 2022;36:251–265. doi: 10.1016/j.idc.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntosh K, Hirsch MS, Bloom A. COVID-19: Epidemiology, virology, and prevention. 16 May 2022. UpToDate. [cited 12 June 2022]. In: UpToDate. [Google Scholar]
- 4.Salzberger B, Buder F, Lampl B, Ehrenstein B, Hitzenbichler F, Holzmann T, Schmidt B, Hanses F. Epidemiology of SARS-CoV-2. Infection. 2021;49:233–239. doi: 10.1007/s15010-020-01531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez MA, Franco S. Impact of COVID-19 in liver disease progression. Hepatol Commun . 2021;5:1138–1150. doi: 10.1002/hep4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Lei J, Li Z, Yan L. Potential effects of coronaviruses on the liver: An update. Front Med. 2021;8:651658. doi: 10.3389/fmed.2021.651658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark R, Waters B, Stanfill AG. Elevated liver function tests in COVID-19: Causes, clinical evidence, and potential treatments. Nurse Pract. 2021;46:21–26. doi: 10.1097/01.NPR.0000722316.63824.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 10.Harisha EJ, Gosavi S, Rao AA, Sahana GV, Manjunath S, Meghana TC. Liver: Function and dysfunction in COVID-19. J Family Med Prim Care. 2022;11:758–761. doi: 10.4103/jfmpc.jfmpc_7_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, Teo EK, Ang TL. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol. 2020;19:627–634. doi: 10.1016/j.aohep.2020.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Ouali S, Romero-Marrero C, Regueiro M. Hepatic manifestations of COVID-19 Cleve Clin J Med 2020. [DOI] [PubMed] [Google Scholar]
- 13.Díaz LA, Idalsoaga F, Cannistra M, Candia R, Cabrera D, Barrera F, Soza A, Graham R, Riquelme A, Arrese M, Leise MD, Arab JP. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693–7706. doi: 10.3748/wjg.v26.i48.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu D, Du Q, Yan S, Guo X-G, He Y, Zhu G, Zhao K, Ouyang S. Liver injury in COVID-19: Clinical features and treatment management. Virol J. 2021;18:121. doi: 10.1186/s12985-021-01593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509–519. doi: 10.1177/2050640620924157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fassan M, Mescoli C, Sbaraglia M, Guzzardo V, Russo FP, Fabris R, Trevenzoli M, Pelizzaro F, Cattelan AM, Basso C, Navalesi P, Farinati F, Vettor R, Dei Tos AP. Liver histopathology in COVID-19 patients: A mono-Institutional series of liver biopsies and autopsy specimens. Pathol Res Pract. 2021;221:153451. doi: 10.1016/j.prp.2021.153451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zghal M, Bouhamed M, Mellouli M, Triki M, Kallel R, Ayedi L, Boudawara TS, Makni S. Liver injury in COVID-19: pathological findings. Pan Afr Med J. 2022;41:56. doi: 10.11604/pamj.2022.41.56.31114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radzina M, Putrins DS, Micena A, Vanaga I, Kolesova O, Platkajis A, Viksna L. Post-COVID-19 Liver Injury: Comprehensive Imaging With Multiparametric Ultrasound. J Ultrasound Med. 2022;41:935–949. doi: 10.1002/jum.15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmit G, Lelotte J, Vanhaebost J, Horsmans Y, Van Bockstal M, Baldin P. The Liver in COVID-19-Related Death: Protagonist or Innocent Bystander? Pathobiology. 2021;88:88–94. doi: 10.1159/000512008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar P, Sharma M, Kulkarni A, Rao PN. Pathogenesis of Liver Injury in Coronavirus Disease 2019. J Clin Exp Hepatol. 2020;10:641–642. doi: 10.1016/j.jceh.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saviano A, Wrensch F, Ghany MG, Baumert TF. Liver Disease and Coronavirus Disease 2019: From Pathogenesis to Clinical Care. Hepatology. 2021;74:1088–1100. doi: 10.1002/hep.31684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozano-Sepulveda SA, Galan-Huerta K, Martínez-Acuña N, Arellanos-Soto D, Rivas-Estilla AM. SARS-CoV-2 another kind of liver aggressor, how does it do that? Ann Hepatol. 2020;19:592–596. doi: 10.1016/j.aohep.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lizardo-Thiebaud MJ, Cervantes-Alvarez E, Limon-de la Rosa N, Tejeda-Dominguez F, Palacios-Jimenez M, Méndez-Guerrero O, Delaye-Martinez M, Rodriguez-Alvarez F, Romero-Morales B, Liu WH, Huang CA, Kershenobich D, Navarro-Alvarez N. Direct or Collateral Liver Damage in SARS-CoV-2-Infected Patients. Semin Liver Dis. 2020;40:321–330. doi: 10.1055/s-0040-1715108. [DOI] [PubMed] [Google Scholar]
- 26.Lei HY, Ding YH, Nie K, Dong YM, Xu JH, Yang ML, Liu MQ, Wei L, Nasser MI, Xu LY, Zhu P, Zhao MY. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed Pharmacother. 2021;133:111064. doi: 10.1016/j.biopha.2020.111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guardigni V, Del Turco ER, Badia L, Galli S, Scolz K, Viale P, Verucchi G. Pre-existing HBV, and HCV infections do not affect COVID-19-related outcomes: An observational retrospective study. Hepatitis . 2021;21:5. [Google Scholar]
- 28.Mohammed A, Paranji N, Chen PH, Niu B. COVID-19 in Chronic Liver Disease and Liver Transplantation: A Clinical Review. J Clin Gastroenterol. 2021;55:187–194. doi: 10.1097/MCG.0000000000001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driggers KE, Sadowski BW, Shagla E, Kwok RM. Care of the Hepatology Patient in the COVID-19 Era. Curr Hepatol Rep. 2022;21:9–20. doi: 10.1007/s11901-021-00581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kariyawasam JC, Jayarajah U, Abeysuriya V, Riza R, Seneviratne SL. Involvement of the Liver in COVID-19: A Systematic Review. Am J Trop Med Hyg. 2022;106:1026–1041. doi: 10.4269/ajtmh.21-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulkarni AV, Tevethia HV, Premkumar M, Arab JP, Candia R, Kumar K, Kumar P, Sharma M, Rao PN, Reddy DN. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vranić L, Radovan A, Poropat G, Mikolašević I, Milić S. Non-Alcoholic Fatty Liver Disease and COVID-19-Two Pandemics Hitting at the Same Time. Medicina (Kaunas) 2021;57 doi: 10.3390/medicina57101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhury T, Sultana J, Dutta J, Gousy N, Hassan KN. Rapid Turn From Cirrhosis to Encephalopathy Following COVID-19 Infection: A Cautionary Tale. Cureus. 2022;14:e22089. doi: 10.7759/cureus.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridruejo E, Soza A. The liver in times of COVID-19: What hepatologists should know. Ann Hepatol. 2020;19:353–358. doi: 10.1016/j.aohep.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilead . Food and Drug Administration approves Gilead’s antiviral Veklury® (remdesivir) for treatment of COVID-19. Gilead: Press Releases. 2020 Oct. [cited 20 June 2022] [Google Scholar]
- 36.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 37. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Remdesivir. National Institute of Diabetes and Digestive and Kidney Diseases. Feb, 2022. [cited 20 June 2022]. In: National Library of Medicine . [PubMed] [Google Scholar]
- 38.Phend C. Remdesivir safety forecast: Watch the liver, kidneys. Medpage Today. 2020 May. [cited 17 June 2022]. In: Medpage Today. [Google Scholar]
- 39.Moon AM, Barritt AS. Elevated liver enzymes in patients with COVID-19: Look, but not too hard. Dig Dis Sci. 2021;66:1767–1769. doi: 10.1007/s10620-020-06585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.COVID-19 Treatment Guidelines. Antiviral drugs that are approved, authorized, or under evaluation for treatment of COVID-19. Apr, 2022. [cited 27 June 2022]. Available from: https://www.empr.com/home/news/acetylcysteine-treatment-for-acute-liver-failure-associated-with-remdesivir/
- 41.Meini S, Pagotto A, Longo B, Vendramin I, Pecori D, Tascini C. Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. J Clin Med. 2020;9 doi: 10.3390/jcm9072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y, Mo X, Wang J, Wang Y, Peng P, Chen X, Hong W, Xiao G, Liu J, Zhang L, Hu F, Li F, Zhang F, Deng X, Li L. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med . 2020;1:105–113.e4. doi: 10.1016/j.medj.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang S, Wang R, Li L, Hong D, Ru R, Rao Y, Miao J, Chen N, Wu X, Ye Z, Hu Y, Xie M, Zuo M, Lu X, Qiu Y, Liang T. Liver injury in critically ill and non-critically ill COVID-19 patients: A multicenter, retrospective, observational study. Front Med. 2020;7:347. doi: 10.3389/fmed.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheuplein VA, Seifried J, Malczyk AH, Miller L, Höcker L, Vergara-Alert J, Dolnik O, Zielecki F, Becker B, Spreitzer I, König R, Becker S, Waibler Z, Mühlebach MD. High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus. J Virol. 2015;89:3859–3869. doi: 10.1128/JVI.03607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alavi Darazam I, Shokouhi S, Pourhoseingholi MA, Irvani SSN, Mokhtari M, Shabani M, Amirdosara M, Torabinavid P, Golmohammadi M, Hashemi SP, Azimi A, Maivan MHJ, Rezaei O, Zali A, Hajiesmaeili M, Dehbsneh HS, Kusha AH, Shoushtari MT, Khalili N, Soleymaninia A, Gachkar L, Khoshkar A. Role of interferon therapy in severe COVID-19: The COVIFERON randomized controlled trial. Sci Rep. 2021:11: 8059. doi: 10.1038/s41598-021-86859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, Kazemzadeh H, Yekaninejad MS. A Randomized Clinical Trial of the Efficacy and Safety of Interferon β-1a in Treatment of Severe COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raschi E, Caraceni P, Poluzzi E, De Ponti F. Baricitinib, JAK inhibitors and liver injury: a cause for concern in COVID-19? Expert Opin Drug Saf. 2020;19:1367–1369. doi: 10.1080/14740338.2020.1812191. [DOI] [PubMed] [Google Scholar]
- 49.Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13:1756284820959183. doi: 10.1177/1756284820959183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901–1905. doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alukal JJ, Naqvi HA, Thuluvath PJ. Vaccination in Chronic Liver Disease: An Update. J Clin Exp Hepatol. 2022;12:937–947. doi: 10.1016/j.jceh.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolak S, Said A, German R, Hayney MS, Caldera F. Optimizing Immunization Strategies in Adult Patients With Chronic Liver Disease and Liver Transplant Recipients. Gastroenterol Hepatol. 2022;18:196–206. [PMC free article] [PubMed] [Google Scholar]
- 54.Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, Selzner N, Schiff J, McDonald M, Tomlinson G, Kulasingam V, Kumar D, Humar A. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoo CZ, Tan KC, Abdullah S, Sim BLH, Omar H, Tan SS. Severe hepatocellular liver injury after COVID-19 vaccination without autoimmune hepatitis features: A case series. ACG Case Rep J. 2022;9:pe00760. [Google Scholar]
- 56.Tsoris A, Marlar CA. Use of the Child-Pugh score in liver disease. StatPearls. 2022 Mar. [cited 20 June 2022]. In: National Library of Medicine. [PubMed] [Google Scholar]
- 57.World Health Organization. COVID-19 advice for the public: Getting vaccinated. Apr, 2022. [cited 20 June 2022] [Google Scholar]
- 58.Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, Mutei MA, Abdel-Wareth L, Uyeki TM, Swerdlow DL, Barakat M, Zaki SR. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186:652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elnaggar M, Abomhya A, Elkhattib I, Dawoud N, Doshi R. COVID-19 and liver diseases, what we know so far. World J Clin Cases. 2022;10:3969–3980. doi: 10.12998/wjcc.v10.i13.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russo FP, Burra P, Zanetto A. COVID-19 and liver disease: where are we now? Nat Rev Gastroenterol Hepatol. 2022;19:277–278. doi: 10.1038/s41575-022-00607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartl L, Haslinger K, Angerer M, Jachs M, Simbrunner B, Bauer DJM, Semmler G, Scheiner B, Eigenbauer E, Strassl R, Breuer M, Kimberger O, Laxar D, Trauner M, Mandorfer M, Reiberger T. Age-adjusted mortality and predictive value of liver chemistries in a Viennese cohort of COVID-19 patients. Liver Int. 2022;42:1297–1307. doi: 10.1111/liv.15274. [DOI] [PMC free article] [PubMed] [Google Scholar]