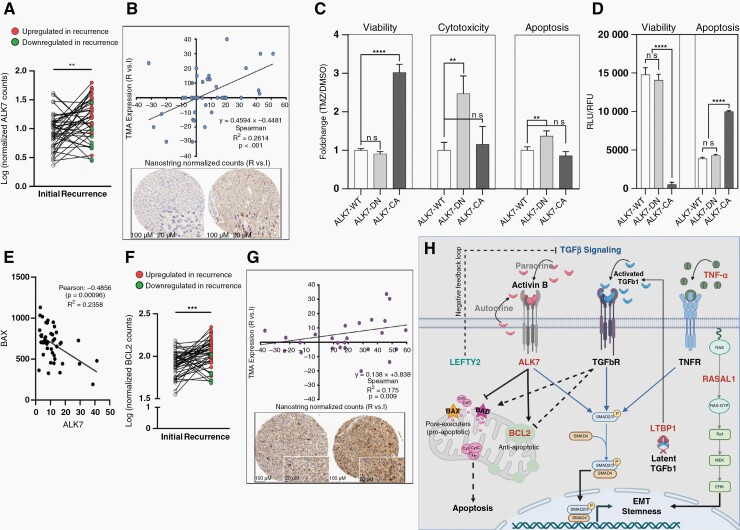
Figure 5.
ALK7 is upregulated in the TMZ-resistant tumor of patients with late recurrence (A) Pairwise comparison of ALK7 mRNA expression. (B) Top: Correlation of tumor-specific ALK7 IHC scores and Nanostring normalized counts from tissue punches. Bottom: Example of TMA punches from the tumor center of initial (left) and recurrent (right) tumor from patient 063 by ALK7 IHC. (C) TMZ response of U87 cells overexpressing ALK7 wild-type (ALK7-WT), dominant negative mutant (ALK7-DN) or constitutive active mutant (ALK7-CA) induced by DOX. Fold change in viability, cytotoxicity and apoptosis following treatment with 100uM TMZ or DMSO is shown after 48 hrs. (D) Viability (relative fluorescent units, RFU) and apoptosis (relative luminescent units, RLU) of cells in the absence of TMZ induced by DOX for 48 hrs. ns (P > .05), ** (P ≤ .01), *** (P ≤ .001). (E) Inverse Pearson correlation of ALK7 and BAX in our cohort of primary and recurrent gliomas. (F) Pairwise comparison of BCL2 mRNA expression. (G) Correlation of BCL2 IHC scores and Nanostring normalized counts from tissue punches (top) and an example of TMA punches from the tumor center of initial (left) and recurrent (right) tumor from patient 027 by BCL2 IHC. (H) Model of concerted TGFβ signaling pathway. ALK7, LTBP1, RASAL1 upregulation and LEFTY2 downregulation translate into increased TGFβ induced EMT and stemness. To overcome TGFβ-induced apoptosis, recurrent tumor cells downregulate pro-apoptotic proteins BAX and BAD and upregulate BCL2 (see E and Supplementary Figure 15). Dashed arrows indicate mechanisms that are uncoupled in recurrent tumors.