Abstract
Macrophages are well known for their phagocytic functions in innate immunity across species. In mammals, they rapidly consume a large amount of energy by shifting their metabolism from mitochondrial oxidative phosphorylation toward aerobic glycolysis, to perform the effective bactericidal function upon infection. Meanwhile, they strive for sufficient energy resources by restricting systemic metabolism. In contrast, under nutrient deprivation, the macrophage population is down-regulated to save energy for survival. Drosophila melanogaster possesses a highly conserved and comparatively simple innate immune system. Intriguingly, recent studies have shown that Drosophila plasmatocytes, the macrophage-like blood cells, adopt comparable metabolic remodeling and signaling pathways to achieve energy reassignment when challenged by pathogens, indicating the conservation of such metabolic strategies between insects and mammals. Here, focusing on Drosophila macrophages (plasmatocytes), we review recent advances regarding their comprehensive roles in local or systemic metabolism under homeostasis or stress, emphasizing macrophages as critical players in the crosstalk between the immune system and organic metabolism from a Drosophila perspective.
Keywords: Macrophage, Drosophila, Immune system, Metabolism, Plasmatocyte
Introduction
The immune system in Drosophila comprises two major arms, the humoral immune response and the cellular immune response, in response to bacterial and fungal infections (Bajgar et al. 2021; Lu et al. 2020). The humoral response includes the induction of antimicrobial peptides (AMPs) via the Toll and IMD (immune deficiency) signaling pathways (Yi et al. 2014). The fat body, a functional homolog of the vertebrate liver, is the primary tissue producing AMPs and secreting them into the body cavity in response to a stimulus (Lemaitre and Hoffmann 2007). The cellular response in Drosophila is performed through two primary mechanisms, i.e., phagocytosis and encapsulation, by circulating blood cells (hemocytes) in the body cavity (Lemaitre and Hoffmann 2007).
In Drosophila, at least three types of blood cells akin to vertebrate myeloid cells have been identified: plasmatocytes, crystal cells, and lamellocytes (Evans et al. 2003; Lan et al. 2020; Mukherjee et al. 2011; P et al. 2020). Plasmatocytes, representing 90–95% of all mature hemocytes, are phagocytes and are very similar to cells of the mammalian innate immune system, in particular macrophages and neutrophils (Bajgar et al. 2021). Crystal cells, which constitute ~ 5% of mature hemocytes, are non-phagocytic but express the oxidoreductase prophenoloxidase (proPO) to mediate the melanization process (Bidla et al. 2007; Mukherjee et al. 2011). Lamellocytes are large, flat cells that primarily function in encapsulating objects too large to be phagocytosed. Unlike plasmatocytes and crystal cells, lamellocytes are not found in embryos or adults, but are only produced in larvae. Furthermore, they are rarely observed in healthy larvae but are rapidly and massively produced upon parasitic wasp infection (Evans et al. 2003; Rizki and Rizki 1992).
These blood cells are sentinels in host defense, especially the macrophage-like plasmatocytes, the phagocytic function of which is highly conserved in the course of the evolution (Bajgar et al. 2021). These immune cells require a large amount of energy to perform their bactericidal function in the critical moment of bacterial infection. In mammals, activated immune cells, such as monocytes, macrophages, and neutrophils, employ aerobic glycolysis to satisfy rapid energy demands (Pearce and Pearce 2013), and accumulate immuno-metabolites to facilitate the antimicrobial response (Rosenberg et al. 2022). Similarly, the Drosophila macrophage-like plasmatocytes adopt comparable metabolic strategies and signaling pathways when challenged by pathogens (Bajgar and Dolezal 2018; Krejcova et al. 2019). In addition, they strive for sufficient energy resources from systemic development and metabolism to supply their immune behaviors (Bajgar et al. 2021). Here, we review the recent advances regarding the comprehensive roles of macrophages in local or systemic metabolism. Since the involved processes in Drosophila and mammals are highly conserved during evolution, the knowledge gained from either Drosophila or mammalian studies would both provide significant insights for fully understanding the metabolic adaptability in immune systems.
Drosophila hematopoiesis
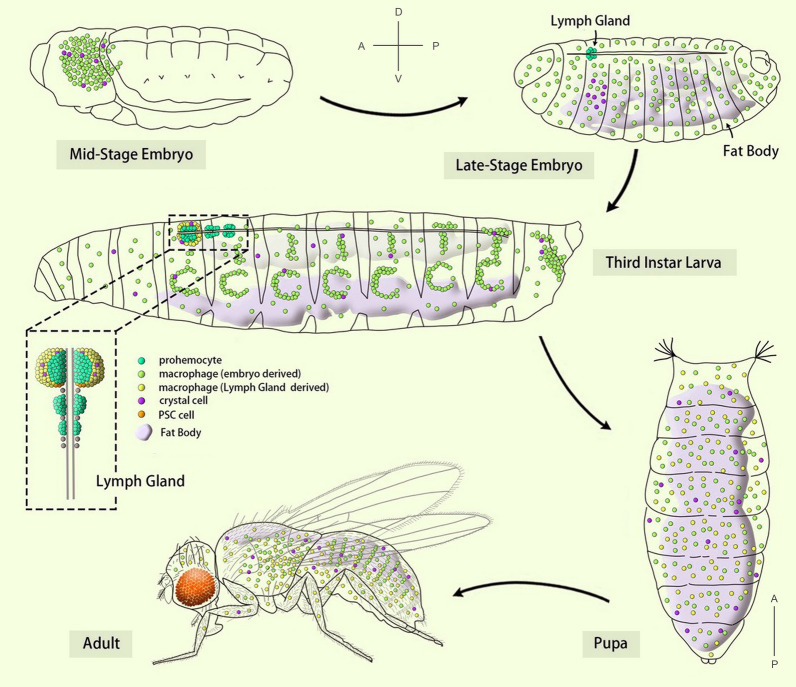
Drosophila melanogaster is a well-established animal model employed to study the hematopoietic processes, through which the immune cells are generated. Like in vertebrates, Drosophila hematopoiesis occurs in two spatially and temporally distinct waves (Fig. 1) (Crozatier and Meister 2007; Evans et al. 2003; Rodrigues et al. 2021). The first wave of Drosophila hematopoiesis occurs during embryogenesis in the head mesoderm and gives rise to approximately 700 plasmatocytes and 30 crystal cells (Tepass et al. 1994; Wood and Jacinto 2007). These embryonically derived blood cells comprise the larval circulatory hemocytes and the sessile pools of blood cells in subcuticular hematopoietic pockets (Holz et al. 2003; Wood and Jacinto 2007). The second wave occurs in the larval stage. In addition to the proliferation of the sessile blood cells in subcuticular hematopoietic pockets, the lymph gland, a specialized hematopoietic organ, contributes mainly to the blood cell population (Makhijani et al. 2011; Morin-Poulard et al. 2021). In the lymph gland, the blood progenitor cells differentiate into mature blood cells, including plasmatocytes, crystal cells, and lamellocytes. At the onset of metamorphosis, the lymph gland is disassociated and the blood cells within it are dispersed into the circulatory system (Grigorian et al. 2011).
Fig. 1.
Drosophila hematopoiesis. The anterior (A)-posterior (P) and dorsal (D)-ventral (V) axes indicate the orientation of embryo/larva/adult, except the pupa is orientated with anterior to the upside and posterior to the downside. PSC posterior signaling center
Drosophila hematopoietic processes have developmental and functional affinities in mammals (Gold and Bruckner 2014). For instance, in humans, RUNX1 is a well-described target of chromosomal translocations leading to acute myeloid leukemia (AML) (Banerjee et al. 2019; Okuda et al. 1996; Speck and Gilliland 2002). Interestingly, the Drosophila ortholog of RUNX1, Lozenge (Lz), is an essential transcription factor for crystal cell specification and development during both waves of hematopoiesis (Bras et al. 2012; Dyer et al. 2017; Lebestky et al. 2000; Miller et al. 2017). Myeloid leukemia factor 1 (MLF1) is another identified target associated with AML (Yoneda-Kato et al. 1996). In flies, MLF is also known to control blood cell development by stabilizing Lz (Miller et al. 2017). Since the hemopoietic system in Drosophila is simpler than that of mammals, its study is a highly efficient way to increase knowledge and understanding of the underlying mechanisms and etiologies of human blood disease.
Macrophages in Drosophila
Macrophages are present in all animals and play diverse roles serving as the front line of protection against invading pathogens (Lim et al. 2017). The most classic role of macrophages is phagocytosis (Franken et al. 2016). In vertebrates, macrophages are known to polarize into bactericidal (M1) or healing (M2) functional phenotypes in response to an activating stimulus (Mills et al. 2000). M1 macrophages are associated with tissue proinflammatory response and microbial killing. M2 macrophages are associated with the resolution of inflammation, wound healing, and are also observed under the conditions of helminth infections and allergies (Breda et al. 2019).
In Drosophila, plasmatocytes are macrophage-like blood cells that are differentiated from the hematopoietic precursors, prohemocytes. The GATA protein Serpent (Srp) is an early marker defining hemocyte precursors in both embryos and larvae (Jung et al. 2005; Rehorn et al. 1996). GATA family proteins are evolutionarily highly conserved transcription factors first identified in hematopoietic and cardiac development (Tremblay et al. 2018). Srp regulates the expression of glide/glial cells missing (gcm) and gcm2, which are critical lineage-specific transcription factors that direct the specification and terminal differentiation of embryonic plasmatocytes (Alfonso and Jones 2002; Bernardoni et al. 1997). Their effects on larval hematopoiesis, however, remain equivocal because neither is expressed in the larval lymph gland or in circulating hemocytes (Avet-Rochex et al. 2010; Bataille et al. 2005; Lebestky et al. 2000).
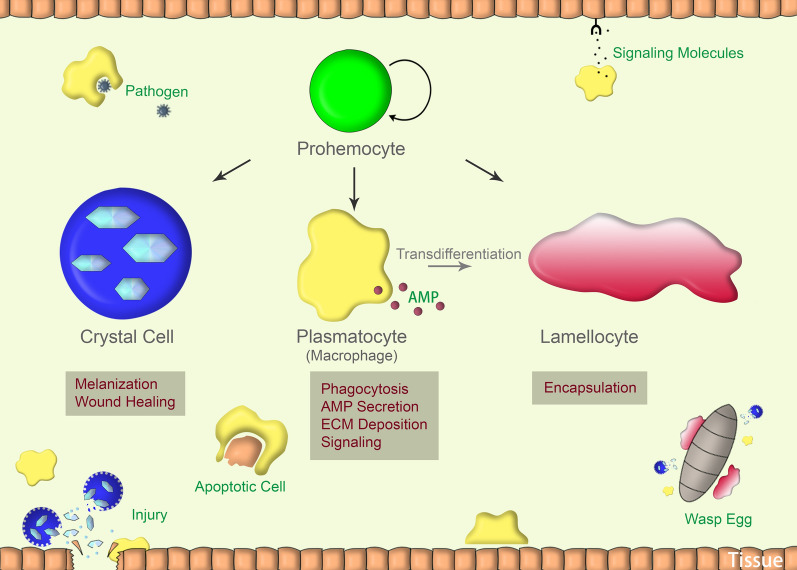
Plasmatocytes constitute the largest population of hemocytes in Drosophila and show significant functional diversity (Fig. 2). Studies in the last two decades have revealed parallel multi-functions of Drosophila plasmatocytes, as counterparts of macrophages in mammals. In Drosophila, beyond many developmental functions, plasmatocytes play diverse pivotal roles during the innate immune response, including maintenance of tissue or organ homeostasis, monitoring tissue damage, recognition of apoptotic cells and, most importantly, defense against invading pathogens (Babcock et al. 2008; Bunt et al. 2010; Franc et al. 1996; Goto et al. 2003; Kierdorf et al. 2020; Mase et al. 2021; Olofsson and Page 2005; Wood and Martin 2017). In addition, plasmatocytes have the potential to trans-differentiate into lamellocytes in response to parasitic infection (Stofanko et al. 2010).
Fig. 2.
Drosophila hemocytes and their functions. ECM extracellular matrix, AMP antimicrobial peptides
In mammals, macrophages are an extremely heterogeneous population corresponding to their diverse roles (Gordon and Pluddemann 2017). Drosophila plasmatocytes have long been regarded as a homogeneous population. However, multiple studies using single-cell RNA sequencing have recently revealed their heterogeneity (Cattenoz et al. 2020, 2021; Coates et al. 2021; Hartenstein 2020; Mase et al. 2021; Tattikota et al. 2020). In particular, Shin et al. (2020) reported distinctive subpopulations of plasmatocytes in embryonically derived circulating and sessile hemocytes at the larval stage, based on the expression of Peroxidasin (Pxn) and Hemolectin (Hml), two commonly used markers for plasmatocytes. Although the expression of these two markers are mostly overlap in these blood cells, subpopulations of plasmatocytes with only Hml or Pxn expression are also observed. These subtypes of plasmatocytes could be specialized to function in the different aspects of homeostasis, including immunity and metabolism. Interestingly, when Hml-positive (Hml +) hemocytes are killed upon the expression of pro-apoptotic genes, the number of remaining hemocytes (Pxn-positive and Hml-negative [Pxn + Hml-]) is dramatically increased, suggesting the existence of an intrinsic mechanism to maintain a constant population of plasmatocytes during development.
Metabolism in macrophages shifts toward aerobic glycolysis upon infection
In vertebrates, the polarized M1 macrophages undergo an overall change in morphology and utilize aerobic glycolysis—a metabolic pathway of generating adenosine triphosphate (ATP) usually in low-oxygen environments—to quickly provide adequate energy for phagocytosis (Galvan-Pena and O’Neill 2014; O’Neill and Pearce 2016; Pearce and Pearce 2013; Van den Bossche et al. 2017). Aerobic glycolysis takes place in the cytosol and converts glucose into lactate along with the production of 2 ATPs per glucose molecule. This energy yield is much lower than that of the oxidative phosphorylation pathway, which can generate 32 ATPs from the complete oxidation of glucose through the mitochondrial tricarboxylic acid (TCA) cycle (Vaupel and Multhoff 2021). Aerobic glycolysis, however, runs much faster than oxidative phosphorylation due to having far fewer reaction steps (Chandel et al. 2021). Therefore, activated macrophages shift their metabolism toward aerobic glycolysis to meet the sharp demand for energy.
Like in mammalian activated (M1) macrophages, a recent study indicates that Drosophila macrophages (plasmatocytes) also undertake a dramatic shift toward aerobic glycolysis upon infection by pathogens (Krejcova et al. 2019). Such an evolutionary conserved metabolic reprogramming in macrophages is mediated by hypoxia-inducible factor 1 alpha (HIF-1α, known as Sima in Drosophila) (Corcoran and O'Neill 2016; Krejcova et al. 2019) (Fig. 3; Table 1). It is well-known that HIF-1α functions as a major regulator in response to hypoxia. HIF-1α is unstable and quickly degrades in normoxia, while in hypoxia HIF-1α becomes stabilized and is translocated into nuclei where it functions as a transcription factor to activate target genes including a set of aerobic glycolysis genes (Centanin et al. 2005; Iommarini et al. 2017). During the acute phase of infection, HIF-1α activity is increased in Drosophila macrophages (Krejcova et al. 2019). Previous studies have reported that nuclear factor kappa B (NF-ĸB) facilitates the stabilization of HIF-1α under normal oxygen levels in Drosophila and mice (Jung et al. 2003; van Uden et al. 2011). It is speculated that infection-induced NF-ĸB signaling promotes the normoxic stabilization of HIF-1α in activated macrophages (Dolezal et al. 2019). Increased HIF-1α levels enhance the transcription of a series of glycolytic genes. In particular, as a target of HIF-1α, lactate dehydrogenase (LDH) activity is elevated in macrophages upon infection to catalyze the conversion of pyruvate into lactate (Firth et al. 1995; Krejcova et al. 2019). In contrast, knocking down HIF-1α decreases the expression of aerobic glycolysis genes in activated macrophages, which leads to the metabolic preference of oxidative phosphorylation and the failure to resist infection (Krejcova et al. 2019). Therefore, HIF-1α stabilization upon infection is a crucial event to elicit the metabolic switch toward aerobic glycolysis for rapid energy production to support effective phagocytosis.
Fig. 3.
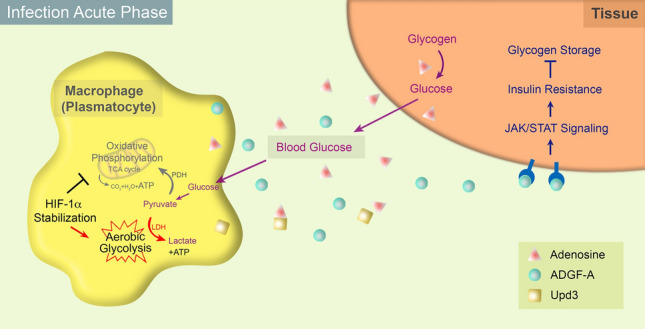
Macrophages alter local and systemic metabolisms upon infection in Drosophila. Upon infection, HIF-1α stabilization in activated macrophages locally promotes the metabolic switch toward the aerobic glycolysis for rapid energy production to support the effective phagocytosis. Pyruvate, the key metabolite of glucose, is generally transported into mitochondria and is converted into acetyl-CoA by pyruvate dehydrogenase (PDH) for participating in the TCA cycle. In the acute phase of infection, the mitochondrial transport of pyruvate and the following ATP production by oxidative phosphorylation are repressed. Instead, cytosolic pyruvate is converted into lactate by lactate dehydrogenase (LDH) coupled with high-speed ATP production. At the same time, the activated macrophages restrict systemic metabolisms to grab more nutrients by secreting multiple effectors. The extracellular adenosine released by macrophages is recognized or taken up by other tissues, promoting glucose production from glycogen stores and glucose release into the hemolymph. This process is negatively controlled by adenosine deaminase-related growth factor A (ADGF-A), which is also produced by macrophages and catalyzes the degradation of adenosine. As another important effector secreted from macrophages, Upd3/IL-6 amplifies JAK/STAT signaling in other tissues reducing their insulin sensitivity and glycogen storage
Table 1.
List of key factors influencing metabolism directly or indirectly in Drosophila
| Factors | Type of molecules | Sources | Targets | Functions | References |
|---|---|---|---|---|---|
| HIF-1α | Transcription regulator | Macrophages | Macrophages | Converts the metabolic strategy to aerobic glycolysis | Krejčová et al. (2019) |
| Adenosine | Nucleotide | Macrophages | Organism | Reflects the nutritional demands and regulates the systemic metabolism | Bajgar and Dolezal (2018); Bajgar et al. (2015) |
| ADGF-A | Extracellular deaminase | Macrophages | Organism | Catalyzes the degradation of adenosine | Bajgar and Dolezal (2018); |
| Dolezal et al. (2005) | |||||
| Upd3 | Cytokine | Macrophages | Organism | Causes systemic insulin resistance | Woodcock et al. (2015) |
| Edin | Secreted peptide | Fat Body | Macrophages | Up-regulates sessile macrophage population and promotes their release into the circulatory system | Vanha-Aho et al. (2015) |
| NimB5 | Adipokine | Fat Body | Macrophages | Reduces the proliferation of macrophages upon nutrient scarcity | Ramond et al. (2020) |
| Pvf3 | PDGF-family growth factor | Macrophages | Fat body | Contributes to lipid storage | Cox et al. (2021) |
| Pvr | PDGF/VEGF receptor | Fat Body | Fat body | Contributes to lipid storage | Cox et al. (2021) |
Another significant issue of this metabolic switch is the remodeling of mitochondrial metabolism since the TCA cycle is inactivated (Nagao et al. 2019). In M1 macrophage mitochondria, the interruption in the TCA cycle causes the accumulation of metabolic intermediates, including succinate, which attenuates the degradation of HIF-1α by prolyl hydroxylases, leading to the stabilization of HIF-1 α (Tannahill et al. 2013). In addition, mitochondria undergo continuous fission and fusion to dynamically control mitochondrial quality and function (Ray et al. 2021). The M1 macrophage is characterized by mitochondrial fission, whereas M2 macrophages that utilize oxidative phosphorylation to generate ATP have elongated mitochondria (Breda et al. 2019). In Drosophila, mitochondrial fission–fusion events contribute to blood progenitor differentiation and the eventual blood cell count (Ray et al. 2021). However, it is unclear whether Drosophila macrophage activation upon infection is also coupled with the dynamic changes of mitochondrial morphology.
Alteration of systemic metabolism by macrophages upon infection
Macrophages lack significant nutritional storage, which means they cannot sustain persistent activation (Bajgar et al. 2021). To meet the sudden nutritional requirements during the effective immune response, the innate immune system not only converts metabolic processes in activated macrophages for rapid energy production, but also seizes nutrients as fuel from non-immune systems (Bajgar et al. 2015) (Fig. 3).
Upon infection, the activated macrophages release multiple types of signaling molecules, including adenosine, to increase nutrient uptake thereby supporting their enhanced energy requirement (Bajgar and Dolezal 2018; Bajgar et al. 2015). As an intracellular purine metabolite, an elevated adenosine concentration is typically a consequence of cellular metabolic status, such as the increased consumption of ATP (Eltzschig 2013). Upon infection by wasp eggs or bacteria, activated blood cells release adenosine into the extracellular compartment. Through adenosine receptors or transporters, the adenosine signal received in non-immune systems, such as the brain, imaginal discs, and fat bodies, inhibits their metabolic activities to save nutrients for immune cells. In the main storage organ fat body, the extracellular adenosine inhibits glycogen synthesis through AMPK, promotes the production of glucose from glycogen stores and thereby facilitates the release of glucose into the hemolymph (Aymerich et al. 2006; Bajgar and Dolezal 2018; Zuberova et al. 2010). The extracellular adenosine also suppresses the growth and development processes in larvae to free up energy for the immune response, whereas the mutant larvae that lack the adenosine receptor develop normally but fail to effectively combat the invasion (Bajgar and Dolezal 2018; Bajgar et al. 2015).
Previous studies have shown that systemic insulin resistance (IR) is able to facilitate the over-production of macrophages, a phenomenon that is conserved from Drosophila to humans (Straub 2014; Woodcock et al. 2015). Insulin is the key player to let blood glucose into cells for consumption to produce energy. In situations of long-term high blood glucose (hyperglycemia), cells may stop responding to insulin, a condition known as insulin resistance. Macrophages present a constitutive expression of JAK/STAT-activating cytokine Unpaired 3 (Upd3) in Drosophila or interleukin-6 (IL-6) in humans (Kierdorf et al. 2020), which have been considered as the critical factor inducing insulin resistance. JAK/STAT is an evolutionarily conserved signaling pathway as a critical regulator in the immune system and in a wide range of developmental and metabolic processes (Arbouzova and Zeidler 2006; Dodington et al. 2018; Ferguson and Martinez-Agosto 2017; Villarino et al. 2017). Upon infection, an increased amount of Upd3/IL-6 secreted from the activated macrophage population amplifies JAK/STAT signaling in muscles, the main reservoir for stored glycogen, reducing their insulin sensitivity and thereby suppressing their blood glucose utilization (Mashili et al. 2013; Yang and Hultmark 2017; Yang et al. 2015). Consequently, systemic insulin resistance increases the circulating glucose thus enabling the cells of the immune system to produce more energy.
Dynamic control of metabolism in macrophages during infection
After the acute phase of the immune response, the rapid amplification of macrophages is not needed. Therefore the energy balance between the immune and non-immune systems must be re-built, otherwise the persistent high-energy cost may adversely affect the survival of the organism (Demas 2004). In addition, the nutrients reallocated to the immune cells could be utilized by the pathogens to cause worsening of the infection (Bajgar and Dolezal 2018; Passalacqua et al. 2016). For example, Listeria monocytogenes, a facultative intracellular bacterial pathogen found in the cytosol of Drosophila phagocytic cells, acquires nutrients from the host cell to support its growth and replication (Mansfield et al. 2003; Passalacqua et al. 2016). Likewise, energy for antibacterial macrophages may directly nourish the pathogens, through which the host survival is decreased (Bajgar and Dolezal 2018). Thus, organisms require proper regulation for energy allocation to avoid excessive consumption of energy.
Indeed, when the infection is cleared, the metabolic strategies adopted by activated macrophages can be reversed, for example the termination of aerobic glycolysis (Krejcova et al. 2019). During the resolution phase of infection, the immune system has killed the majority of the pathogens, the bacterial residues are cleared, and homeostasis is re-established in the host. During this period, macrophages become quiescent again, switching back to basal metabolism and prepare to re-switch their metabolic processes when faced with the next challenge by a pathogen (Mosser and Edwards 2008; Pearce and Pearce 2013). Besides the autonomous metabolic switch, the systemic metabolic alteration upon infection is negatively controlled by adenosine deaminase-related growth factor A (ADGF-A) (Bajgar and Dolezal 2018). As an adenosine-degrading enzyme, ADGF-A is expressed during the late-stage of infection and catalyzes the irreversible deamination of adenosine to inosine (Dolezal et al. 2005). Ablating ADGF-A expression in macrophages prevents degradation of adenosine, leading to prolonged energy reallocation to the immune system, glycogen storage reduction, and sometimes increased amounts of intracellular pathogens due to the availability of excessive energy (Bajgar and Dolezal 2018).
Metabolic crosstalk between macrophages and systemic tissues
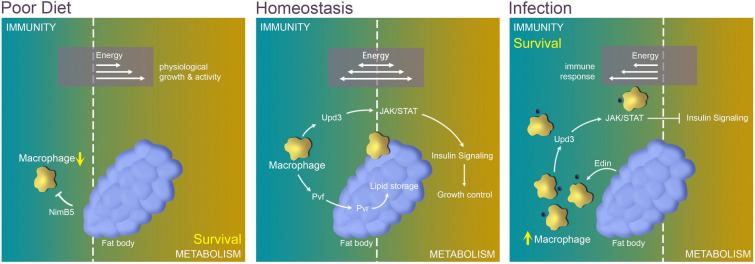
Immunity and metabolism are intimately linked (Chambers et al. 2012; Chen and Huang 2022; Lee and Lee 2018). Organisms maintain a sophisticated energy allocation between metabolic consumption and the immune system under homeostasis. However, when external or internal stressors, such as infection or starvation, destroy this equilibrium, extra energy is directed to the party with priority demand (Fig. 4) (Bajgar et al. 2015; Banerjee et al. 2019; Kraaijeveld and Godfray 1997; van der Most et al. 2011). To date, the most studied metabolic organs that reveal complex interplay with macrophages in Drosophila are fat bodies and muscles.
Fig. 4.
Macrophage-associated interplay between metabolism and the immune system in Drosophila. During homeostasis, macrophages are maintained in a basal number and at a basal metabolic level, playing essential roles to regulate lipid storage and organism growth through Pvf/Pvr signaling and JAK/STAT signaling. However, under stress conditions due to infection or nutrition deficiency, the immune system and other tissues compete for energy metabolism. Upon infection, the energy is reassigned to the immune system by inhibiting systemic metabolisms, which supports the rapid amplification of the macrophage population to defend against the pathogens. Likewise, when the nutrition is limited, the energy is reallocated toward the metabolic processes to ensure the survivability of the organism, and the number of macrophages is suppressed to save nutrients
Homeostatic condition
During homeostasis, the macrophage population is maintained at a low number with a basal level of energy demand. Surprisingly, the unspecific ablation of blood cells in larvae under normal conditions dramatically reduces adult growth with typical markers of the insulin resistance (P et al. 2020), suggesting a requirement for macrophages in the regulation of physiological processes.
It has been reported that Drosophila macrophages generate a PDGF/VEGF-family growth factor, Pvf3, to facilitate lipid storage in the larval fat body (Cox et al. 2021). Both pvf3 mutant larvae and hemocyte-specific pvf3 deficient larvae exhibit over 50% reduction in triglyceride content and smaller fat body cell size than in control larvae. The PDGF/VEGF receptor Pvr (also called Vegfr/Stasis) located in fat body cells transduces Pvf signaling to mediate lipid storage in the fat body (Hoch and Soriano 2003; Zheng et al. 2017). Blocking Pvr specifically in the fat body has a very similar effect to that observed in pvf3 deficient larvae (Cox et al. 2021). It is noteworthy that, PDGFcc, the mouse ortholog of Pvf3, secreted by macrophages, is also essential for lipid storage (Cox et al. 2021).
In healthy Drosophila muscles, macrophage-derived Upd3 is required as a metabolic regulator via activating JAK/STAT signaling (Kierdorf et al. 2020). When JAK/STAT signaling is suppressed in larval muscles, reduced activity of AKT, a critical kinase to convey insulin signaling (Roth et al. 2018), and reduced glycogen levels are observed (Yang and Hultmark 2017). Interestingly, another study reported that the loss of the JAK/STAT receptor Domeless in adult muscle tissue leads to hyper-activation of AKT, accompanied by decreased muscle function and a shorter lifespan (Kierdorf et al. 2020). The disparity of these results is possibly due to the different developmental stages investigated. Nevertheless, the macrophage-derived cytokine signal must be received in muscles to regulate AKT-insulin signaling activity and metabolic homeostasis.
Infection
As described above, upon infection, energy is reallocated to the immune system to support a rapid and efficient response to kill pathogens. The increased number of macrophages secret more Upd3 or adenosine, which systemically activates JAK/STAT signaling and adenosine-associated signaling, respectively, to inhibit metabolic processes (Kim et al. 2013; Mashili et al. 2013; Pean et al. 2017; Woodcock et al. 2015). On the other hand, the immune system also profits from the activities of other tissue/organs. For example, in Drosophila larvae infected by parasitoid wasps, sessile macrophage populations are up-regulated and released into the circulation via a fat body-secreted peptide known as Edin (Vanha-Aho et al. 2015).
Similar to the infection situation, blood cells tend to amplify rapidly when a subtype of plasmatocyte is specifically ablated, to maintain a sufficient number of immune cells. In response to this expansion demand, the remaining plasmatocytes produce more Upd3, which activates JAK/STAT signaling and decreases insulin signaling in the fat body, leading to less lipid storage in fat body cells (Shin et al. 2020).
Nutrient-rich/deficiency condition
In addition to stimulation by pathogenic organisms, the nutrient condition is another critical factor affecting the immune system. Similar to conditions when infected by pathogens, mammalian macrophages also remodel their metabolic strategy to aerobic glycolysis under lipid-rich conditions (Bajgar et al. 2021). In contrast, the metabolic strategy of Drosophila macrophages under chronic lipid-rich conditions has yet to be investigated. However, it has been found that a chronic lipid-rich diet in Drosophila induces the production of Upd3 by macrophages causing insulin insensitivity and a reduced lifespan via activation of the JAK/STAT pathway (Woodcock et al. 2015). In contrast, under nutrient-deficient conditions, scarce resources need to be reassigned to critical tissues and to support essential processes such as development (Ramond et al. 2020). Meanwhile, the number of macrophages is down-regulated to a basal level to save energy (Dolezal et al. 2019). Fat body-derived adipokine NimB5 binds to macrophages to reduce their proliferation in conditions of nutrient scarcity (Ramond et al. 2020). Blocking NimB5 could result in the production of numerous macrophages, which is likely to deplete energy stores and eventually leads to mortality of larvae raised on a poor diet (Ramond et al. 2020).
Conclusion and perspectives
Drosophila is a powerful model organism to investigate immunity and metabolism due to its comparatively simple immune/hematopoietic or metabolic systems and the conservation of functional mechanisms throughout the evolution (Graham and Pick 2017; Ratheesh et al. 2015; Zhao et al. 2021). In this review, we have discussed the critical role of macrophages in local or systemic metabolism under homeostasis or stress in Drosophila. During homeostasis, macrophages are maintained in a basal number and at a basal metabolic level, playing essential roles in metabolism and growth control. However, under stress conditions, for example due to infection or nutrition deficiency, the immune system and other tissues compete for energy and nutrients. When a sudden infection occurs, the number of macrophages is rapidly amplified to defend the host organism against the pathogen (P et al. 2020). In this situation, the immune system has the priority energy demand for organism survival, and metabolism is inevitably affected to save energy resources for the immune system. Likewise, when the nutrition intake is limited, to ensure the survivability of the organism, metabolic processes are primarily supplied and energy demand for macrophages is suppressed. In short, the organism controls the energy flow between the immune system and organic metabolism such that the more important process is prioritized according to the nature of the stress (Fig. 4, Table 2).
Table 2.
Summary of metabolism alterations in Drosophila upon infection or starvation
| Healthy situation | Infection | Poor diet | |
|---|---|---|---|
| Number of macrophages | Maintained at a physiological level | Massively increased | Decreased |
| Energy demand of macrophages | At a basal level | High energy consumption | Lower than a basal/normal level |
| Metabolic pathways of macrophages | Oxidative phosphorylation | Aerobic glycolysis | |
| HIF-1α in macrophages | Rapidly degraded | Stabilized | |
| Upd3 from macrophages | At a constitutive level | Increased | |
| JAK/STAT signaling in tissues | At a constitutive level | Amplified | |
| Systemic insulin resistance | OFF | ON | |
| Interaction between macrophages and fat body | Macrophages generate Pvf3 to facilitate the routine lipid storage in fat body | Adenosine from macrophages inhibits glycogen synthesis in fat body; Edin from fat body promotes the release of sessile macrophages into the circulatory system | Fat body-derived adipokine NimB5 binds to macrophages to reduce their proliferation |
Macrophages are highly plastic cells that play multiple roles in organisms. They are also closely associated with many human diseases (Wynn et al. 2013). Morbid obesity, tumors, and infection could cause chronic inflammation induced by continuous macrophage activation and subsequent metabolic disturbances such as insulin resistance (Musselman et al. 2011; Porporato 2016). Indeed, tumors and obese adipose tissues are infiltrated by a large number of macrophages (Geeraerts et al. 2017; Jia et al. 2014; Roth et al. 2018; Weisberg et al. 2003). Tumor-associated macrophages (TAMs) existing in the tumor microenvironment play a pivotal role in supporting tumor growth. The metabolic profile of TAMs is likely biased toward the M2 phenotype (Halbrook et al. 2019). In response to the altered tumor microenvironment, they also present a metabolic switch toward glycolysis to produce altered cytokines and angiogenic factors thereby supporting tumor growth and survival (Puthenveetil and Dubey 2020). Therefore, the regulation of metabolic programs in macrophages could be a therapeutic target to improve the treatment of human diseases.
Macrophages, however, are not the only cell type to perform the phagocytic function. Epithelial follicle cells may serve as phagocytes in the Drosophila ovary (Lebo et al. 2021). Glial cells phagocytize apoptotic neuronal cells in the central nervous system (Hilu-Dadia et al. 2018). Moreover, macrophages and glial cells share the same transcription factor Gcm for their development (Trebuchet et al. 2019). It would be interesting to investigate whether these cells undergo the same metabolic shift for performing their phagocytic function.
Acknowledgements
This work was supported by the grants of the National Natural Science Foundation of China (31970475 and 32170832 to YS, 31970506 and 32170541 to LZ), and the Fundamental Research Funds for Central Universities, China (202012004 to LZ).
Author contributions
WL and SL: wrote the manuscript; WL: drew the figures; FZ: helped improve the writing; LZ and YS: improved and revised the manuscript.
Data availability
This article does not contain any original research data.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Animal and human rights statement
No animal or human rights are involved in this article.
Footnotes
Wang Luo and Sumin Liu contributed equally to the study.
Contributor Information
Long Zhao, Email: zhaolong@ouc.edu.cn.
Ying Su, Email: suying@ouc.edu.cn.
References
- Alfonso TB, Jones BW. Gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev Biol. 2002;248:369–383. doi: 10.1006/dbio.2002.0740. [DOI] [PubMed] [Google Scholar]
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Avet-Rochex A, Boyer K, Polesello C, Gobert V, Osman D, Roch F, Auge B, Zanet J, Haenlin M, Waltzer L. An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis. BMC Dev Biol. 2010;10:65. doi: 10.1186/1471-213X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymerich I, Foufelle F, Ferre P, Casado FJ, Pastor-Anglada M. Extracellular adenosine activates AMP-dependent protein kinase (AMPK) J Cell Sci. 2006;119:1612–1621. doi: 10.1242/jcs.02865. [DOI] [PubMed] [Google Scholar]
- Babcock DT, Brock AR, Fish GS, Wang Y, Perrin L, Krasnow MA, Galko MJ. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc Natl Acad Sci USA. 2008;105:10017–10022. doi: 10.1073/pnas.0709951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar A, Dolezal T. Extracellular adenosine modulates host-pathogen interactions through regulation of systemic metabolism during immune response in Drosophila. PLoS Pathog. 2018;14:e1007022. doi: 10.1371/journal.ppat.1007022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar A, Krejcova G, Dolezal T. Polarization of macrophages in insects: opening gates for immuno-metabolic research. Front Cell Dev Biol. 2021;9:629238. doi: 10.3389/fcell.2021.629238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar A, Kucerova K, Jonatova L, Tomcala A, Schneedorferova I, Okrouhlik J, Dolezal T. Extracellular adenosine mediates a systemic metabolic switch during immune response. PLoS Biol. 2015;13:e1002135. doi: 10.1371/journal.pbio.1002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U, Girard JR, Goins LM, Spratford CM. Drosophila as a genetic model for hematopoiesis. Genetics. 2019;211:367–417. doi: 10.1534/genetics.118.300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille L, Auge B, Ferjoux G, Haenlin M, Waltzer L. Resolving embryonic blood cell fate choice in Drosophila: interplay of GCM and RUNX factors. Development. 2005;132:4635–4644. doi: 10.1242/dev.02034. [DOI] [PubMed] [Google Scholar]
- Bernardoni R, Vivancos V, Giangrande A. Glide/gcm is expressed and required in the scavenger cell lineage. Dev Biol. 1997;191:118–130. doi: 10.1006/dbio.1997.8702. [DOI] [PubMed] [Google Scholar]
- Bidla G, Dushay MS, Theopold U. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci. 2007;120:1209–1215. doi: 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- Bras S, Martin-Lanneree S, Gobert V, Auge B, Breig O, Sanial M, Yamaguchi M, Haenlin M, Plessis A, Waltzer L. Myeloid leukemia factor is a conserved regulator of RUNX transcription factor activity involved in hematopoiesis. Proc Natl Acad Sci USA. 2012;109:4986–4991. doi: 10.1073/pnas.1117317109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breda CNS, Davanzo GG, Basso PJ, Saraiva Camara NO, Moraes-Vieira PMM. Mitochondria as central hub of the immune system. Redox Biol. 2019;26:101255. doi: 10.1016/j.redox.2019.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt S, Hooley C, Hu N, Scahill C, Weavers H, Skaer H. Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev Cell. 2010;19:296–306. doi: 10.1016/j.devcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattenoz PB, Monticelli S, Pavlidaki A, Giangrande A. Toward a consensus in the repertoire of hemocytes identified in Drosophila. Front Cell Dev Biol. 2021;9:643712. doi: 10.3389/fcell.2021.643712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattenoz PB, Sakr R, Pavlidaki A, Delaporte C, Riba A, Molina N, Hariharan N, Mukherjee T, Giangrande A. Temporal specificity and heterogeneity of Drosophila immune cells. EMBO J. 2020;39:e104486. doi: 10.15252/embj.2020104486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanin L, Ratcliffe PJ, Wappner P. Reversion of lethality and growth defects in Fatiga oxygen-sensor mutant flies by loss of hypoxia-inducible factor-alpha/Sima. EMBO Rep. 2005;6:1070–1075. doi: 10.1038/sj.embor.7400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MC, Song KH, Schneider DS. Listeria monocytogenes infection causes metabolic shifts in Drosophila melanogaster. PLoS ONE. 2012;7:e50679. doi: 10.1371/journal.pone.0050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS. Glycolysis. Cold Spring Harb Perspect Biol. 2021;13:a040535. doi: 10.1101/cshperspect.a040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Huang X. Cytosolic lipolysis in non-adipose tissues: energy provision and beyond. FEBS J. 2022 doi: 10.1111/febs.16161. [DOI] [PubMed] [Google Scholar]
- Coates JA, Brooks E, Brittle AL, Armitage EL, Zeidler MP, Evans IR. Identification of functionally distinct macrophage subpopulations in Drosophila. Elife. 2021;10:e58686. doi: 10.7554/eLife.58686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran SE, O'Neill LA. HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N, Crozet L, Holtman IR, Loyher PL, Lazarov T, White JB, Mass E, Stanley ER, Elemento O, Glass CK, et al. Diet-regulated production of PDGFcc by macrophages controls energy storage. Science. 2021;373:eabe9383. doi: 10.1126/science.abe9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–1126. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- Demas GE. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm Behav. 2004;45:173–180. doi: 10.1016/j.yhbeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dodington DW, Desai HR, Woo M. JAK/STAT - emerging players in metabolism. Trends Endocrinol Metab. 2018;29:55–65. doi: 10.1016/j.tem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Dolezal T, Dolezelova E, Zurovec M, Bryant PJ. A role for adenosine deaminase in Drosophila larval development. PLoS Biol. 2005;3:e201. doi: 10.1371/journal.pbio.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal T, Krejcova G, Bajgar A, Nedbalova P, Strasser P. Molecular regulations of metabolism during immune response in insects. Insect Biochem Mol Biol. 2019;109:31–42. doi: 10.1016/j.ibmb.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Dyer JO, Dutta A, Gogol M, Weake VM, Dialynas G, Wu X, Seidel C, Zhang Y, Florens L, Washburn MP, et al. Myeloid leukemia factor acts in a chaperone complex to regulate transcription factor stability and gene expression. J Mol Biol. 2017;429:2093–2107. doi: 10.1016/j.jmb.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK. Extracellular adenosine signaling in molecular medicine. J Mol Med (berl) 2013;91:141–146. doi: 10.1007/s00109-013-0999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. doi: 10.1016/S1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- Ferguson GB, Martinez-Agosto JA. The TEAD family transcription factor Scalloped regulates blood progenitor maintenance and proliferation in Drosophila through PDGF/VEGFR receptor (Pvr) signaling. Dev Biol. 2017;425:21–32. doi: 10.1016/j.ydbio.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem. 1995;270:21021–21027. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity. 1996;4:431–443. doi: 10.1016/S1074-7613(00)80410-0. [DOI] [PubMed] [Google Scholar]
- Franken L, Schiwon M, Kurts C. Macrophages: sentinels and regulators of the immune system. Cell Microbiol. 2016;18:475–487. doi: 10.1111/cmi.12580. [DOI] [PubMed] [Google Scholar]
- Galvan-Pena S, O'Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraerts X, Bolli E, Fendt SM, Van Ginderachter JA. Macrophage metabolism as therapeutic target for cancer, atherosclerosis, and obesity. Front Immunol. 2017;8:289. doi: 10.3389/fimmu.2017.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold KS, Bruckner K. Drosophila as a model for the two myeloid blood cell systems in vertebrates. Exp Hematol. 2014;42:717–727. doi: 10.1016/j.exphem.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Pluddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15:53. doi: 10.1186/s12915-017-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto A, Kadowaki T, Kitagawa Y. Drosophila hemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects. Dev Biol. 2003;264:582–591. doi: 10.1016/j.ydbio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Graham P, Pick L. Drosophila as a model for diabetes and diseases of insulin resistance. Curr Top Dev Biol. 2017;121:397–419. doi: 10.1016/bs.ctdb.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorian M, Mandal L, Hartenstein V. Hematopoiesis at the onset of metamorphosis: terminal differentiation and dissociation of the Drosophila lymph gland. Dev Genes Evol. 2011;221:121–131. doi: 10.1007/s00427-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbrook CJ, Pontious C, Kovalenko I, Lapienyte L, Dreyer S, Lee HJ, Thurston G, Zhang Y, Lazarus J, Sajjakulnukit P, et al. Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metab. 2019;29:e1396. doi: 10.1016/j.cmet.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V. One too many: the surprising heterogeneity of Drosophila macrophages. EMBO J. 2020;39:e105199. doi: 10.15252/embj.2020105199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilu-Dadia R, Hakim-Mishnaevski K, Levy-Adam F, Kurant E. Draper-mediated JNK signaling is required for glial phagocytosis of apoptotic neurons during Drosophila metamorphosis. Glia. 2018;66:1520–1532. doi: 10.1002/glia.23322. [DOI] [PubMed] [Google Scholar]
- Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- Holz A, Bossinger B, Strasser T, Janning W, Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–4962. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- Iommarini L, Porcelli AM, Gasparre G, Kurelac I. Non-canonical mechanisms regulating hypoxia-inducible factor 1 alpha in cancer. Front Oncol. 2017;7:286. doi: 10.3389/fonc.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, et al. Hepatocyte toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:3878. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. Biochem J. 2003;370:1011–1017. doi: 10.1042/bj20021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Hersperger F, Sharrock J, Vincent CM, Ustaoglu P, Dou J, Gyoergy A, Gross O, Siekhaus DE, Dionne MS. Muscle function and homeostasis require cytokine inhibition of AKT activity in Drosophila. Elife. 2020;9:51595. doi: 10.7554/eLife.51595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Choi SE, Ha ES, Jung JG, Han SJ, Kim HJ, Kim DJ, Kang Y, Lee KW. IL-6 induction of TLR-4 gene expression via STAT3 has an effect on insulin resistance in human skeletal muscle. Acta Diabetol. 2013;50:189–200. doi: 10.1007/s00592-011-0259-z. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld AR, Godfray HC. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. [DOI] [PubMed] [Google Scholar]
- Krejcova G, Danielova A, Nedbalova P, Kazek M, Strych L, Chawla G, Tennessen JM, Lieskovska J, Jindra M, Dolezal T, et al. Drosophila macrophages switch to aerobic glycolysis to mount effective antibacterial defense. Elife. 2019;8:e50414. doi: 10.7554/eLife.50414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Liu S, Zhao L, Su Y. Regulation of Drosophila hematopoiesis in lymph gland: from a developmental signaling point of view. Int J Mol Sci. 2020;21:5246. doi: 10.3390/ijms21155246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- Lebo DPV, Chirn A, Taylor JD, Levan A, Doerre Torres V, Agreda E, Serizier SB, Lord AK, Jenkins VK, McCall K. An RNAi screen of the kinome in epithelial follicle cells of the Drosophila melanogaster ovary reveals genes required for proper germline death and clearance. G3 (Bethesda) 2021;11:066. doi: 10.1093/g3journal/jkaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Lee WJ. Immune-metabolic interactions during systemic and enteric infection in Drosophila. Curr Opin Insect Sci. 2018;29:21–26. doi: 10.1016/j.cois.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lim JJ, Grinstein S, Roth Z. Diversity and versatility of phagocytosis: roles in innate immunity, tissue remodeling, and homeostasis. Front Cell Infect Microbiol. 2017;7:191. doi: 10.3389/fcimb.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Su F, Li Q, Zhang J, Li Y, Tang T, Hu Q, Yu XQ. Pattern recognition receptors in Drosophila immune responses. Dev Comp Immunol. 2020;102:103468. doi: 10.1016/j.dci.2019.103468. [DOI] [PubMed] [Google Scholar]
- Makhijani K, Alexander B, Tanaka T, Rulifson E, Bruckner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–5391. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield BE, Dionne MS, Schneider DS, Freitag NE. Exploration of host-pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell Microbiol. 2003;5:901–911. doi: 10.1046/j.1462-5822.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- Mase A, Augsburger J, Bruckner K. Macrophages and their organ locations shape each other in development and homeostasis - a Drosophila perspective. Front Cell Dev Biol. 2021;9:630272. doi: 10.3389/fcell.2021.630272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashili F, Chibalin AV, Krook A, Zierath JR. Constitutive STAT3 phosphorylation contributes to skeletal muscle insulin resistance in type 2 diabetes. Diabetes. 2013;62:457–465. doi: 10.2337/db12-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Chen A, Gobert V, Auge B, Beau M, Burlet-Schiltz O, Haenlin M, Waltzer L. Control of RUNX-induced repression of Notch signaling by MLF and its partner DnaJ-1 during Drosophila hematopoiesis. PLoS Genet. 2017;13:e1006932. doi: 10.1371/journal.pgen.1006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- Morin-Poulard I, Tian Y, Vanzo N, Crozatier M. Drosophila as a model to study cellular communication between the hematopoietic niche and blood progenitors under homeostatic conditions and in response to an immune stress. Front Immunol. 2021;12:719349. doi: 10.3389/fimmu.2021.719349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T, Kim WS, Mandal L, Banerjee U. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science. 2011;332:1210–1213. doi: 10.1126/science.1199643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Sukumar Hathiramani S, Cagan RL, Baranski TJ. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech. 2011;4:842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. 2019;20:238. doi: 10.3390/ijms20020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/S0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- Olofsson B, Page DT. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 2005;279:233–243. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- P P, Tomar A, Madhwal S, Mukherjee T. Immune control of animal growth in homeostasis and nutritional stress in Drosophila. Front Immunol. 2020;11:1528. doi: 10.3389/fimmu.2020.01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passalacqua KD, Charbonneau ME, O'Riordan MXD. Bacterial metabolism shapes the host-pathogen interface. Microbiol Spectr. 2016;4:10.1128/microbiolspec.VMBF-0027-2015. doi: 10.1128/microbiolspec.VMBF-0027-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pean CB, Schiebler M, Tan SW, Sharrock JA, Kierdorf K, Brown KP, Maserumule MC, Menezes S, Pilatova M, Bronda K, et al. Regulation of phagocyte triglyceride by a STAT-ATG2 pathway controls mycobacterial infection. Nat Commun. 2017;8:14642. doi: 10.1038/ncomms14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveetil A, Dubey S. Metabolic reprograming of tumor-associated macrophages. Ann Transl Med. 2020;8:1030. doi: 10.21037/atm-20-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramond E, Petrignani B, Dudzic JP, Boquete JP, Poidevin M, Kondo S, Lemaitre B. The adipokine NimrodB5 regulates peripheral hematopoiesis in Drosophila. FEBS J. 2020;287:3399–3426. doi: 10.1111/febs.15237. [DOI] [PubMed] [Google Scholar]
- Ratheesh A, Belyaeva V, Siekhaus DE. Drosophila immune cell migration and adhesion during embryonic development and larval immune responses. Curr Opin Cell Biol. 2015;36:71–79. doi: 10.1016/j.ceb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Ray A, Kamat K, Inamdar MS. A conserved role for Asrij/OCIAD1 in progenitor differentiation and lineage specification through functional interaction with the regulators of mitochondrial dynamics. Front Cell Dev Biol. 2021;9:643444. doi: 10.3389/fcell.2021.643444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehorn KP, Thelen H, Michelson AM, Reuter R. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–4031. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol. 1992;16:103–110. doi: 10.1016/0145-305X(92)90011-Z. [DOI] [PubMed] [Google Scholar]
- Rodrigues D, Renaud Y, VijayRaghavan K, Waltzer L, Inamdar MS. Differential activation of JAK-STAT signaling reveals functional compartmentalization in Drosophila blood progenitors. Elife. 2021;10:e61409. doi: 10.7554/eLife.61409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G, Riquelme S, Prince A, Avraham R. Immunometabolic crosstalk during bacterial infection. Nat Microbiol. 2022;7:497–507. doi: 10.1038/s41564-022-01080-5. [DOI] [PubMed] [Google Scholar]
- Roth SW, Bitterman MD, Birnbaum MJ, Bland ML. Innate immune signaling in Drosophila blocks insulin signaling by uncoupling PI(3,4,5)P3 production and Akt activation. Cell Rep. 2018;22:2550–2556. doi: 10.1016/j.celrep.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Cha N, Koranteng F, Cho B, Shim J. Subpopulation of macrophage-like plasmatocytes attenuates systemic growth via JAK/STAT in the Drosophila fat body. Front Immunol. 2020;11:63. doi: 10.3389/fimmu.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck NA, Gilliland DG. Core-binding factors in haematopoiesis and leukaemia. Nat Rev Cancer. 2002;2:502–513. doi: 10.1038/nrc840. [DOI] [PubMed] [Google Scholar]
- Stofanko M, Kwon SY, Badenhorst P. Lineage tracing of lamellocytes demonstrates Drosophila macrophage plasticity. PLoS ONE. 2010;5:e14051. doi: 10.1371/journal.pone.0014051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH. Insulin resistance, selfish brain, and selfish immune system: an evolutionarily positively selected program used in chronic inflammatory diseases. Arthritis Res Ther. 2014;16:S4. doi: 10.1186/ar4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattikota SG, Cho B, Liu Y, Hu Y, Barrera V, Steinbaugh MJ, Yoon SH, Comjean A, Li F, Dervis F, Hung RJ, Nam JW, Ho Sui S, Shim J, Perrimon N. A single-cell survey of Drosophila blood. Elife. 2020;9:e54818. doi: 10.7554/eLife.54818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Fessler LI, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994;120:1829–1837. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- Trebuchet G, Cattenoz PB, Zsamboki J, Mazaud D, Siekhaus DE, Fanto M, Giangrande A. The Repo homeodomain transcription factor suppresses hematopoiesis in Drosophila and preserves the glial fate. J Neurosci. 2019;39:238–255. doi: 10.1523/JNEUROSCI.1059-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Sanchez-Ferras O, Bouchard M. GATA transcription factors in development and disease. Development. 2018;145:dev164384. doi: 10.1242/dev.164384. [DOI] [PubMed] [Google Scholar]
- Van den Bossche J, O'Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- van der Most PJ, de Jong B, Parmentier HK, Verhulst S. Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct Ecol. 2011;25:74–80. doi: 10.1111/j.1365-2435.2010.01800.x. [DOI] [Google Scholar]
- van Uden P, Kenneth NS, Webster R, Muller HA, Mudie S, Rocha S. Evolutionary conserved regulation of HIF-1beta by NF-kappaB. PLoS Genet. 2011;7:e1001285. doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanha-Aho LM, Anderl I, Vesala L, Hultmark D, Valanne S, Ramet M. Edin expression in the fat body is required in the defense against parasitic wasps in Drosophila melanogaster. PLoS Pathog. 2015;11:e1004895. doi: 10.1371/journal.ppat.1004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599:1745–1757. doi: 10.1113/JP278810. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Kanno Y, O'Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Jacinto A. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat Rev Mol Cell Biol. 2007;8:542–551. doi: 10.1038/nrm2202. [DOI] [PubMed] [Google Scholar]
- Wood W, Martin P. Macrophage functions in tissue patterning and disease: new insights from the fly. Dev Cell. 2017;40:221–233. doi: 10.1016/j.devcel.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock KJ, Kierdorf K, Pouchelon CA, Vivancos V, Dionne MS, Geissmann F. Macrophage-derived upd3 cytokine causes impaired glucose homeostasis and reduced lifespan in Drosophila fed a lipid-rich diet. Immunity. 2015;42:133–144. doi: 10.1016/j.immuni.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Hultmark D. Drosophila muscles regulate the immune response against wasp infection via carbohydrate metabolism. Sci Rep. 2017;7:15713. doi: 10.1038/s41598-017-15940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Kronhamn J, Ekstrom JO, Korkut GG, Hultmark D. JAK/STAT signaling in Drosophila muscles controls the cellular immune response against parasitoid infection. EMBO Rep. 2015;16:1664–1672. doi: 10.15252/embr.201540277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HY, Chowdhury M, Huang YD, Yu XQ. Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol. 2014;98:5807–5822. doi: 10.1007/s00253-014-5792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda-Kato N, Look AT, Kirstein MN, Valentine MB, Raimondi SC, Cohen KJ, Carroll AJ, Morris SW. The t(3;5)(q25.1;q34) of myelodysplastic syndrome and acute myeloid leukemia produces a novel fusion gene, NPM-MLF1. Oncogene. 1996;12:265–275. [PubMed] [Google Scholar]
- Zhao L, Gao F, Gao S, Liang Y, Long H, Lv Z, Su Y, Ye N, Zhang L, Zhao C, Wang X, Song W, Zhang S, Dong B. Biodiversity-based development and evolution: the emerging research systems in model and non-model organisms. Sci China Life Sci. 2021;64:1236–1280. doi: 10.1007/s11427-020-1915-y. [DOI] [PubMed] [Google Scholar]
- Zheng H, Wang X, Guo P, Ge W, Yan Q, Gao W, Xi Y, Yang X. Premature remodeling of fat body and fat mobilization triggered by platelet-derived growth factor/VEGF receptor in Drosophila. FASEB J. 2017;31:1964–1975. doi: 10.1096/fj.201601127R. [DOI] [PubMed] [Google Scholar]
- Zuberova M, Fenckova M, Simek P, Janeckova L, Dolezal T. Increased extracellular adenosine in Drosophila that are deficient in adenosine deaminase activates a release of energy stores leading to wasting and death. Dis Model Mech. 2010;3:773–784. doi: 10.1242/dmm.005389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any original research data.