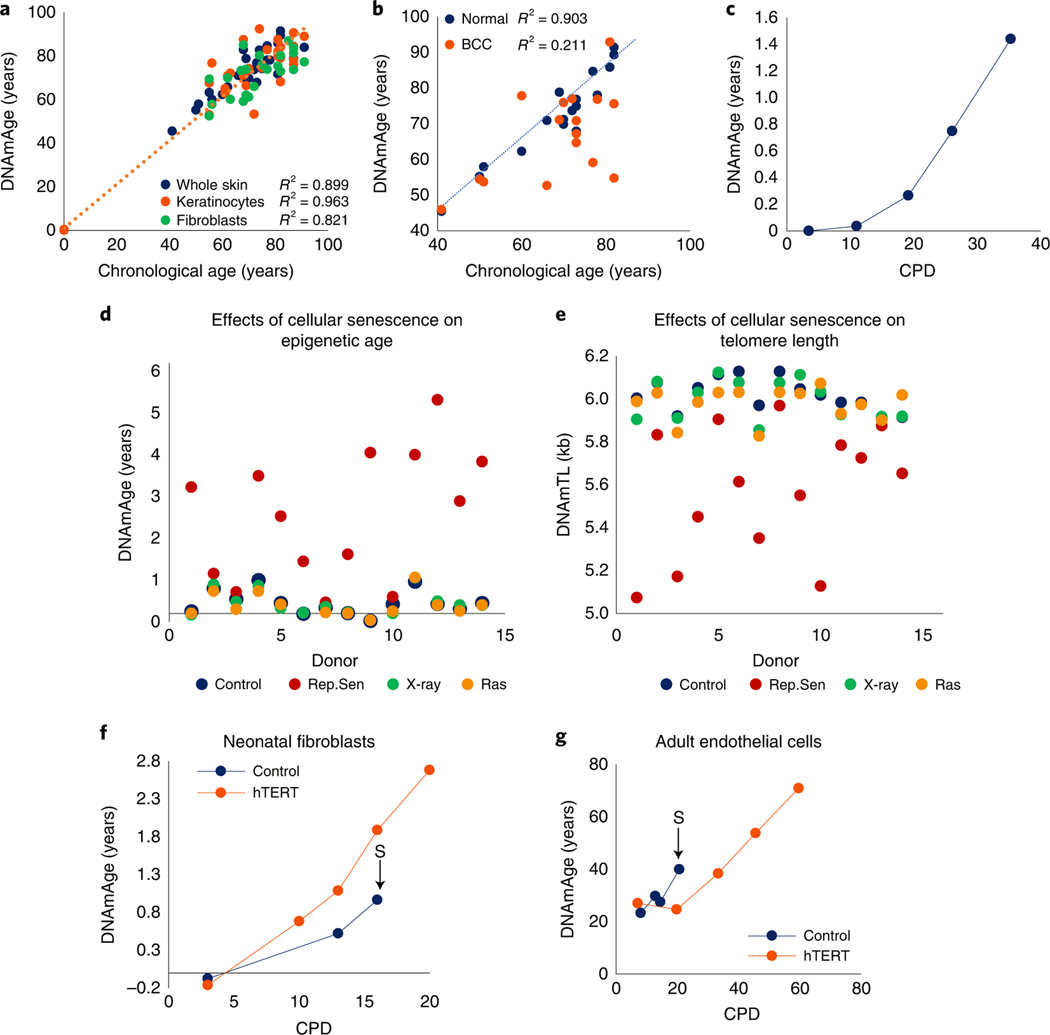
Fig. 1 |. EpiAge is distinct from cellular senescence and telomere attrition.
a, Measurement of EpiAge (DNAmAge) of whole skin, keratinocytes and fibroblasts from 14 healthy human skin samples. Absence of samples between 1 and 40 years old reflects the scarcity of hospital admissions of this demographic group. b, Measurement of DNAmAge of 17 BCCs and corresponding adjacent healthy skin. c, EpiAge of in vitro-cultured neonatal human dermal keratinocytes (HDK) derived from foreskin. CPD, cumulative population doubling. Representative of more than three experiments. d, EpiAges of primary human dermal fibroblasts isolated from skin of 14 healthy neonatal donors and subjected to 20 Gy X-rays, transduced to express oncogenic ras or cultured until replicative senescence (Rep.Sen). e, DNA methylation-based estimation of telomere length (DNAmTL) of cells described in d. f, DNAmAge of neonatal primary human dermal fibroblasts transduced with empty vector (control) or hTERT-expressing vector (hTERT). The arrow and letter ‘S’ denote the point at which the untreated control cells became senescent. Representative of two experiments. g, EpiAge of adult HCAECs transduced with empty vector (control) or vector expressing hTERT. Representative of three experiments