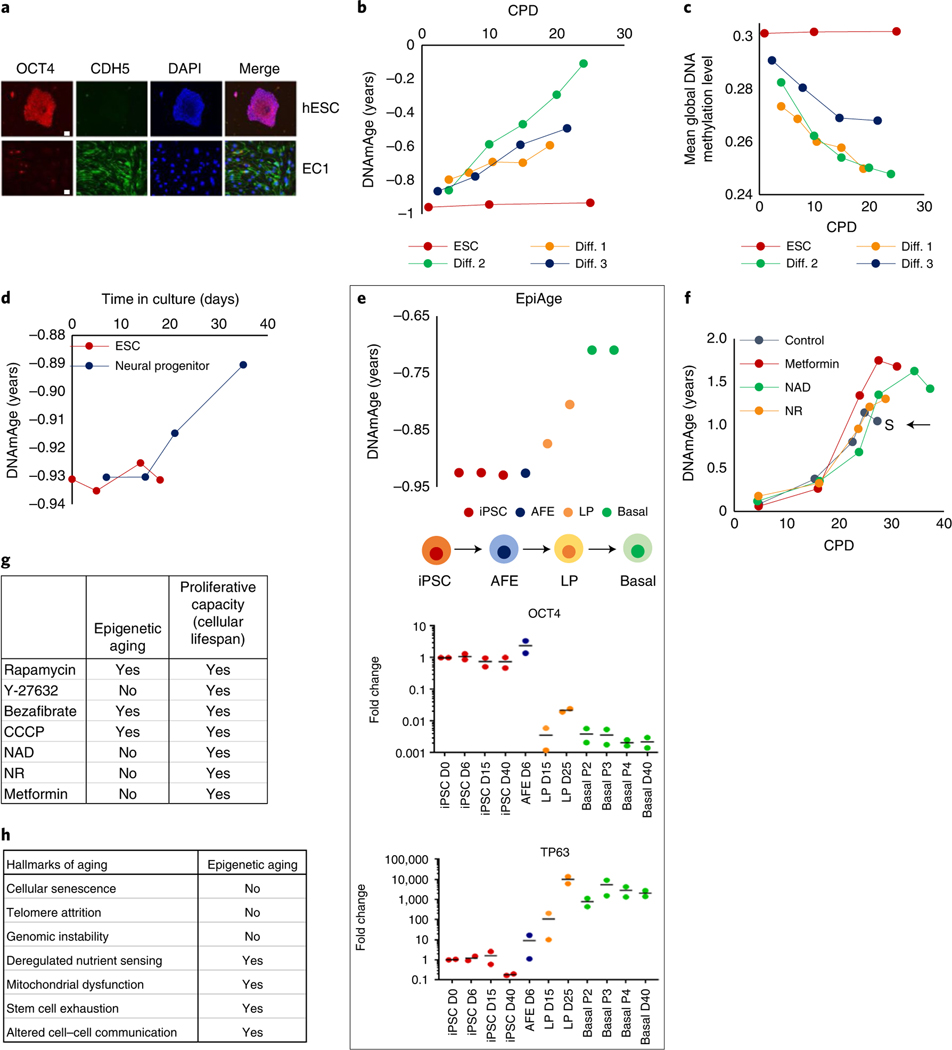
Fig. 4 |. Epigenetic clock starts ticking when ESCs lose their pluripotency.
a, Immunofluorescence analyses of OCT4 (red), expressed exclusively by human ESCs (hESCs) and VE-cadherin (CDH5) in green, a marker of endothelial cells. EC1 cells were differentiated from hESCs to become endothelial cells. Red dots in EC1 are anti-CD31 beads used in endothelial cell purification, which were stained by secondary antibody. Representative of two experiments. Scale bars, 20 μm. b, EpiAge of three independent lines of endothelial cells (Diff.1–3) differentiated from hESCs. c, Mean methylation levels of the cells described in b. d, EpiAge following differentiation of hESCs into neural progenitor cells. Representative of a single experiment. e, Tracking of EpiAge of human iPSCs that were differentiated into human lung epithelial basal cells (basal) via transition through AFE and LP cells. RnA levels of OCT4 and TP63 of these cells were measured by qPCR. Representative of two experiments. D, day; P, passage. f, EpiAge of human neonatal keratinocytes treated continuously with nAD, nR or metformin. The arrow and letter ‘S’ denote when untreated control cells became senescent. Representative of two experiments. g, Comparison of the effects of tested compounds on epigenetic aging and cellular population lifespan. h, Summary of the relationship between the hallmarks of aging and epigenetic aging.