Background:
The increasing focus of population surveillance and research on maternal—and not only fetal and infant—health outcomes is long overdue. The United States maternal mortality rate is higher than any other high-income country, and Georgia is among the highest rates in the country. Severe maternal morbidity (SMM) is conceived of as a “near miss” for maternal mortality, is 50 times more common than maternal death, and efforts to systematically monitor SMM rates in populations have increased in recent years. Much of the current population-based research on SMM has occurred in coastal states or large cities, despite substantial geographical variation with higher maternal and infant health burdens in the Southeast and rural regions.
Methods:
This population-based study uses hospital discharge records linked to vital statistics to describe the epidemiology of SMM in Georgia between 2009 and 2020.
Results:
Georgia had a higher SMM rate than the United States overall (189.2 vs. 144 per 10,000 deliveries in Georgia in 2014, the most recent year with US estimates). SMM was higher among racially minoritized pregnant persons and those at the extremes of age, of lower socioeconomic status, and with comorbid chronic conditions. SMM rates were 5 to 6 times greater for pregnant people delivering infants <1500 grams or <32 weeks’ gestation as compared with those delivering normal weight or term infants. Since 2015, SMM has increased in Georgia.
Conclusion:
SMM represents a collection of life-threatening emergencies that are unevenly distributed in the population and require increased attention. This descriptive analysis provides initial guidance for programmatic interventions intending to reduce the burden of SMM and, subsequently, maternal mortality in the US South.
Key Words: severe maternal morbidity, surveillance, retrospective birth cohort, maternal health
Public health surveillance and research on fetal and infant health outcomes in the United States is longstanding and relatively robust, as evidenced by population-based data systems that utilize vital records and health care data, substantial clinical and population research, and funding. However, recent years have seen a relatively dramatic increase in media reporting of the status of maternal, and not only fetal and infant, health in the United States.1 There have been corresponding calls for increased public health surveillance, research, and action to improve maternal health outcomes and eliminate racial and geographic disparities.2
Growing evidence suggests that the maternal peripartum course should be viewed along a continuum from healthy/uncomplicated to morbid, severely morbid (“near miss”), and fatal.3 The US pregnancy-related mortality ratio in 2018 was 17.3 deaths per 100,000 live births,4 which is substantially higher than the Healthy People 2020 goal of 11.4 per 100,000 live births5 and at least double the number of maternal deaths of other wealthy, developed nations.6 Severe maternal morbidity (SMM), defined as unexpected outcomes of labor and delivery that result in significant short- or long-term consequences to health and conceived of as mortality “near misses”, is ~50 times more prevalent than maternal death in the United States.7
Maternal death is a devastating but fortunately rare outcome. Surveillance and evaluation of SMM may improve opportunities to identify issues of health care access and quality-of-care that contribute to the maternal progression along the peripartum morbidity to mortality continuum.8 Specifically, the measurement and evaluation of trends, causes of, and contributors to SMM can serve as an adjunct to maternal death reviews in the assessment of resource allocation, identification of research priorities, and development of obstetric critical care protocols.9 The study of SMM may also shed light on unique successes and missed opportunities in the management of obstetric conditions.
Unlike maternal mortality or feto-infant morbidity and mortality, which can be monitored through specific population-based surveillance data systems (eg, birth and death certificates), there is no corresponding nationally-uniform data system for SMM. Since the mid-2000s, algorithms have been proposed for identifying probable cases of SMM from hospital discharge forms, often available from state hospital associations.9–11 However, challenges in accessing, managing, and analyzing these administrative data have limited the growth and extent of SMM research, and in many cases, limited the scope of studies to single states or cities. For example, California hospital discharge data has been used to characterize the relationship between SMM and cesarean section,12 short inter-pregnancy interval,13 maternal comorbidities,14 and preterm birth.15 In New York state, Liu, et al16 reported the association between county measures of structural racism and SMM, while in New York City, hospital discharge data has been used to characterize the clinical, social, and hospital-specific correlates of racial and ethnic disparities in SMM.17–19
Research such as that briefly summarized above is critically important for effective action. However, there is a relative paucity of evidence specifically describing the scope, extent, and patterns of SMM in the US South. With only a few exceptions20–22 there is sparse evidence describing the epidemiologic patterns and population burden of SMM in the very states where both overall burden and racial and ethnic disparities are historically largest. For example, the pregnancy-related mortality ratio in Georgia from 2015 to 2017 was 25.1 per 100,000 live births,23 among the highest rates in the United States. This high rate, coupled with wide racial disparities, underscores the need to better understand the distribution and burden of the related but more prevalent construct of SMM in a southeastern US state.
The aim of this study was to provide the descriptive epidemiology of SMM in the state of Georgia for 2009 through 2020, which spans the pre-October and post-October 2015 periods when SMM surveillance changed from ICD-9-CM to ICD-10-CM coding. This descriptive characterization of SMM in Georgia serves to capture critical aspects of the state of maternal health and health care in this racially diverse, populous, southern state. It also serves to galvanize future research in Georgia, the Southeast, and nationally to better understand patterns of SMM, to inform specific actions to reduce SMM, and to eliminate racial, economic, and geographic disparities in SMM. By analyzing the state’s vital statistics and hospital discharge data, the authors sought to assess Georgia’s SMM trends, risk factors, and disparities, laying the groundwork for ongoing surveillance and strategic planning within the state.
MATERIALS AND METHODS
The data source for this study was a retrospective population-based cohort created by deterministically linking the Georgia Discharge Data System (an administrative hospital discharge dataset with International Classification of Disease (ICD) diagnosis and procedure codes associated with hospitalizations) with Georgia Vital Statistics (birth certificates and fetal death records) through a unique identifier for the years 2009 through 2020. The same unique identifier permitted the longitudinal linkage of hospital discharge records to identify SMM events occurring during the delivery hospitalization and during post-delivery rehospitalizations, and thus include SMM events that occur in the postpartum period.
Pregnancies considered “at risk” for an SMM event were at least 22 weeks of gestation among Georgia residents aged 12 to 55 between January 1, 2009 and December 31, 2020, and were recorded on a Georgia hospital discharge record. The pregnancy could have ended in live birth or fetal death; ectopic pregnancies, molar pregnancies, and induced terminations were excluded. Inclusion criteria were a date of conception (determined as delivery date minus clinically estimated gestational age) between July 31, 2008 and June 18, 2020, to allow sufficient follow-up period to observe postpartum SMM events (ie, to include pregnancies at least 22 weeks gestation that were due by January 1, 2009, and to only include pregnancies that were observed through 42 days postpartum in the 2020 data).
SMM events were identified using the 21 diagnoses defined by the Alliance for Innovation of Maternal Health (AIM) (v08-09-2021) and recommended by the CDC.24 For hospital discharges before October 2015, we used the defined ICD-9-CM diagnosis and procedure codes, and for hospital discharges after October 2015, we used the defined ICD-10-CM diagnosis and procedure codes to identify each condition occurring to a woman during her delivery hospitalization. The primary analysis restricts to SMM during the delivery hospitalization, but additional analyses provide estimates combining events from both delivery hospitalization and any rehospitalization SMM event within 42 days of delivery (Supplemental Tables 2–3, Supplemental Digital Content 1, http://links.lww.com/MLR/C588, Supplemental Digital Content 2, http://links.lww.com/MLR/C589).
Blood transfusion of 4 or more units was a defined criterion for SMM as originally proposed by Callaghan,10 but ICD coding does not distinguish the number of units transfused. Therefore, following convention in the SMM literature, we produced estimates of SMM, both including and excluding the blood transfusion criteria to produce more plausible bounds on the true burden of SMM.
To further characterize demographic and pregnancy-related factors associated with SMM, all deliveries were linked through a unique identifier to either live birth or fetal death certificates. From these vital records, we confirmed maternal race/ethnicity and age and added the following to our descriptive characteristics: marital status, education, tobacco use, parity, urbanicity of county of residence, plurality, pre-pregnancy hypertension, gestational hypertension and diabetes, and neonatal outcomes (including gestational age, birth weight, and fetal death vs. live birth).
Observed SMM rate (the number of persons who met severe morbidity criteria per 10,000 delivery hospitalizations) and the crude relative risk of these maternal outcomes according to various maternal sociodemographic and clinical characteristics were calculated. Unadjusted and jointly adjusted SMM rate ratios were estimated using log-binomial regression. We recognize that racial and ethnic disparities in SMM are substantial and are not a product of biologically essential traits but instead of the complex interplay of life course experience shaped by historical and contemporary structural racism. Therefore, we chose to summarize multiply adjusted rate ratios stratified by race/ethnicity for the 2 largest groups in Georgia: non-Hispanic Black and non-Hispanic White. These stratified analyses permit the examination of differences in the association of risk factors with SMM by race/ethnicity.
In the main analysis, adjusted rate ratios were calculated using all years of data. Because social and health care disruptions from the COVID-19 pandemic in 2020 may have influenced either the measurement or occurrence of SMM, we estimate pooled and adjusted results excluding data from 2020 as a sensitivity analysis. Data analyses were performed using R.25 The Emory Institutional Review Board approved all components of this study.
RESULTS
Within the hospital discharge dataset, there were 1,522,047 delivery hospitalizations between 2009 and 2020 among pregnant persons aged 12 to 55 years old. Of these deliveries, 1,472,345 (97%) were among those residing in Georgia and 1,291,246 (88%) of this set were linked with a unique vital statistic record (1,285,291 live births, 5560 fetal deaths, and 395 with a record in both live births and fetal deaths). After exclusion based on gestational age at delivery and date of conception outside of the eligibility window, there were 1,172,752 deliveries included in the analytic cohort.
Among this cohort, 20,701 individuals met the criteria for SMM during delivery hospitalization, including blood transfusion, and 8144 met the criteria excluding transfusion (Table 1).
TABLE 1.
Maternal Sociodemographic and Clinical Characteristics for Severe Maternal Morbidity With and Without Blood Transfusion During Delivery Hospitalization Among Georgia Resident Women Ages 12–55 Years With Delivery Hospitalizations Between 2009–2020
| Including blood transfusion | Excluding blood transfusion | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Delivery hospitalizations, n (%) | SMM events, n (%) | SMM rate | Crude RR (95% CI) | SMM events, n (%) | SMM rate | Crude RR (95% CI) |
| N=1,172,752 | N=20,701 | — | — | N=8,144 | — | — | |
| Race and ethnicity | |||||||
| NH White | 543,294 (46) | 7,252 (35) | 133.5 | Ref | 3,115 (38) | 57.3 | Ref |
| NH Black | 415,138 (35) | 9,742 (47) | 234.7 | 1.8 (1.7, 1.8) | 3,718 (46) | 89.6 | 1.6 (1.5, 1.6) |
| NH Asian | 42,041 (4) | 655 (3) | 155.8 | 1.2 (1.1, 1.3) | 243 (3) | 57.8 | 1.0 (0.9, 1.1) |
| NH Other | 1,764 (0) | 40 (0) | 226.8 | 1.7 (1.2, 2.3) | 14 (0) | 79.4 | 1.4 (0.8, 2.2) |
| NH Multiracial | 26,847 (2) | 457 (2) | 170.2 | 1.3 (1.2, 1.4) | 194 (2) | 72.3 | 1.3 (1.1, 1.5) |
| Hispanic | 131,050 (11) | 2,334 (11) | 178.1 | 1.3 (1.3, 1.4) | 767 (9) | 58.5 | 1.0 (0.9, 1.1) |
| Missing | 12,618 (1) | 221 (1) | 175.1 | — | 93 (1) | 73.7 | — |
| Maternal age | |||||||
| <17 | 12,062 (1) | 308 (1) | 255.3 | 1.6 (1.4, 1.8) | 94 (1) | 77.9 | 1.3 (1.0, 1.6) |
| 17–19 | 82,580 (7) | 1,724 (8) | 208.8 | 1.3 (1.2, 1.4) | 587 (7) | 71.1 | 1.2 (1.1, 1.3) |
| 20–24 | 288,014 (25) | 5,078 (25) | 176.3 | 1.1 (1.1, 1.2) | 1,762 (22) | 61.2 | 1.0 (0.9, 1.1) |
| 25–29 | 334,328 (29) | 5,301 (26) | 158.6 | Ref | 2,026 (25) | 60.6 | Ref |
| 30–34 | 286,419 (24) | 4,612 (22) | 161.0 | 1 (1.0, 1.1) | 1,957 (24) | 68.3 | 1.1 (1.1, 1.2) |
| 35–39 | 137,472 (12) | 2,772 (13) | 201.6 | 1.3 (1.2, 1.3) | 1,260 (15) | 91.7 | 1.5 (1.4, 1.6) |
| >=40 | 31,877 (3) | 906 (4) | 284.2 | 1.8 (1.7, 1.9) | 458 (6) | 143.7 | 2.4 (2.1, 2.6) |
| Marital status | |||||||
| Unmarried | 551,152 (47) | 11,554 (56) | 209.6 | 1.4 (1.4, 1.5) | 4,185 (51) | 75.9 | 1.2 (1.1, 1.3) |
| Married | 619,513 (53) | 9,071 (44) | 146.4 | Ref | 3,921 (48) | 63.3 | Ref |
| Missing | 2,087 (0) | 76 (0) | 364.2 | — | 38 (0) | 182.1 | — |
| Insurance payor | |||||||
| Private | 487,579 (42) | 6,908 (33) | 141.7 | Ref | 3,023 (37) | 62.0 | Ref |
| Medicaid | 571,689 (49) | 11,685 (56) | 204.4 | 1.4 (1.4, 1.5) | 4,182 (51) | 73.2 | 1.2 (1.1, 1.2) |
| Medicare | 15,331 (1) | 398 (2) | 259.6 | 1.8 (1.7, 2.0) | 215 (3) | 140.2 | 2.3 (2.0, 2.6) |
| Self pay | 31,264 (3) | 714 (3) | 228.4 | 1.6 (1.5, 1.7) | 239 (3) | 76.4 | 1.2 (1.1, 1.4) |
| Other payors | 64,925 (6) | 970 (5) | 149.4 | 1.1 (1.0, 1.1) | 469 (6) | 72.2 | 1.2 (1.1, 1.3) |
| Missing | 1,964 (0) | 26 (0) | 132.4 | — | 16 (0) | 81.5 | — |
| Education | |||||||
| Less than high school | 34,501 (3) | 695 (3) | 201.4 | 1.3 (1.2, 1.4) | 236 (3) | 68.4 | 1.1 (0.9, 1.2) |
| Some high school | 133,031 (11) | 2,976 (14) | 223.7 | 1.5 (1.4, 1.5) | 1,010 (12) | 75.9 | 1.2 (1.1, 1.3) |
| High school degree/GED | 350,282 (30) | 6,855 (33) | 195.7 | 1.3 (1.3, 1.3) | 2,546 (31) | 72.7 | 1.1 (1.1, 1.2) |
| Some college or higher | 624,703 (53) | 9,477 (46) | 151.7 | Ref | 4,006 (49) | 64.1 | Ref |
| Missing | 30,235 (3) | 698 (3) | 230.9 | — | 346 (4) | 114.4 | — |
| Tobacco use during pregnancy | |||||||
| Yes | 64,911 (6) | 1,116 (5) | 171.9 | 1.0 (0.9, 1.0) | 446 (5) | 68.7 | 1.0 (0.9, 1.1) |
| No | 1,102,674 (94) | 19,408 (94) | 176.0 | Ref | 7,612 (93) | 69.0 | Ref |
| Missing | 5,167 (0%) | 177 (1) | 342.6 | — | 86 (1) | 166.4 | — |
| NCHS rural-urban | |||||||
| Large central metro | 114,252 (10) | 2,470 (12) | 216.2 | Ref | 846 (10) | 74.0 | Ref |
| Large fringe metro | 548,478 (47) | 9,776 (47) | 178.2 | 0.8 (0.8, 0.9) | 3,690 (45) | 67.3 | 0.9 (0.8, 1.0) |
| Medium metro | 127,893 (11) | 1,692 (8) | 132.3 | 0.6 (0.6, 0.7) | 877 (11) | 68.6 | 0.9 (0.8, 1.0) |
| Small metro | 182,437 (16) | 2,792 (13) | 153.0 | 0.7 (0.7, 0.7) | 1,145 (14) | 62.8 | 0.8 (0.8, 0.9) |
| Micropolitan | 118,127 (10) | 2,361 (11) | 199.9 | 0.9 (0.9, 1.0) | 961 (12) | 81.4 | 1.1 (1.0, 1.2) |
| Non-core | 81,565 (7) | 1,610 (8) | 197.4 | 0.9 (0.9, 1.0) | 625 (8) | 76.6 | 1.0 (0.9, 1.1) |
| Plurality | |||||||
| Multiple | 21,097 (2) | 1,272 (6) | 602.9 | 3.6 (3.4, 3.8) | 389 (5) | 184.4 | 2.7 (2.5, 3.0) |
| Single | 1,151,474 (98) | 19,428 (94) | 168.7 | Ref | 7,754 (95) | 67.3 | Ref |
| Missing | 181 | — | — | — | — | — | — |
| Parity | |||||||
| Nulliparity | 476,055 (41) | 8,706 (42) | 182.9 | Ref | 3,600 (44) | 75.6 | Ref |
| Low multiparity (1–3) | 637,379 (54) | 10,265 (50) | 161.1 | 0.9 (0.9, 0.9) | 3,935 (48) | 61.7 | 0.8 (0.8, 0.9) |
| Grand multipara (4+) | 59,305 (5) | 1,729 (8%) | 291.5 | 1.6 (1.5, 1.7) | 608 (7) | 102.5 | 1.4 (1.2, 1.5) |
| Missing | 13 | — | — | — | — | — | |
| Prenatal care | |||||||
| Prenatal care | 947,472 (81) | 15,676 (76) | 165.5 | Ref | 6,046 (74) | 63.8 | Ref |
| Late or no prenatal care | 60,842 (5) | 1,358 (7) | 223.2 | 1.3 (1.3, 1.4) | 462 (6) | 75.9 | 1.2 (1.1, 1.3) |
| Missing | 164,438 (14) | 3,667 (18) | 223.0 | — | 1,636 (20) | 99.5 | — |
| Pre-pregnancy hypertension | |||||||
| Yes | 21,989 (2) | 795 (4) | 361.5 | 2.1 (2.0, 2.2) | 421 (5) | 191.5 | 2.9 (2.6, 3.2) |
| No | 1,132,578 (97) | 19,523 (94) | 172.4 | Ref | 7,557 (93) | 66.7 | Ref |
| Missing | 18,185 (2) | 383 (2) | 210.6 | — | 166 (2) | 91.3 | — |
| Gestational hypertension | |||||||
| Yes | 64,408 (5) | 2,863 (14) | 444.5 | 2.8 (2.7, 2.9) | 1,476 (18) | 229.2 | 3.8 (3.6, 4.1) |
| No | 1,090,159 (93) | 17,455 (84) | 160.1 | Ref | 6,502 (80) | 59.6 | Ref |
| Missing | 18,185 (2) | 383 (2) | 210.6 | — | 166 (2) | 91.3 | — |
| Gestational diabetes | |||||||
| Yes | 45,790 (4) | 926 (4) | 202.2 | 1.2 (1.1, 1.2) | 391 (5) | 85.4 | 1.2 (1.1, 1.4) |
| No | 1,108,776 (95) | 19,392 (94) | 174.9 | Ref | 7,587 (93) | 68.4 | Ref |
| Missing | 18,186 (2) | 383 (2) | 210.6 | — | 166 (2) | 91.3 | — |
Rate per 10,000 delivery hospitalizations.
counts less than 10 censored with dashed line.
SMM indicates severe maternal morbidity.
Persons at higher risk of SMM during the delivery hospitalization were younger than 19, or 35 years of age and older, belonged to a racially or ethnically minoritized group, lacked a college education, were unmarried, and were uninsured or had coverage other than private health insurance (such as Medicaid or Medicare) (Table 1). Women were also at higher risk of SMM if they had pre-pregnancy or gestational hypertension or diabetes. While the risk of SMM is born unevenly by maternal race and ethnicity, the magnitude of Black-White racial disparities varied across demographic and clinical risk factors (Table 2, Fig. 1). For example, there is nearly no racial difference in SMM among the youngest mothers, but the magnitude of disparity increases with maternal age.
TABLE 2.
Multiply Adjusted Associations Between Individual Covariates and Severe Maternal Mortality With and Without Blood Transfusion During Delivery Hospitalization by Race and Ethnicity, Georgia, 2009–2020
| Delivery hospitalization SMM | Delivery hospitalization SMM, excluding blood transfusion | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | Non-Hispanic Black | Non-Hispanic White | Non-Hispanic Black | |||||
| Characteristic | SMM Rate* | Adjusted RR | SMM Rate* | Adjusted RR | SMM Rate* | Adjusted RR | SMM Rate* | Adjusted RR |
| Race-specific rate | 133.5 | — | 234.7 | — | 57.3 | — | 89.6 | — |
| Maternal age | ||||||||
| <17 | 250.5 | 1.3 (1.0, 1.7) | 257.0 | 1.2 (1.0, 1.4) | 87.7 | 1.2 (0.7, 1.8) | 74.1 | 0.8 (0.5, 1.1) |
| 17–19 | 166.0 | 1.0 (0.9, 1.2) | 238.4 | 1.1 (1.0, 1.2) | 51.0 | 0.8 (0.6, 0.9) | 89.7 | 1.0 (0.9, 1.2) |
| 20–24 | 136.5 | 1.0 (0.9, 1.1) | 214.1 | 1.0 (0.9, 1.1) | 50.7 | 0.9 (0.8, 1.0) | 74.1 | 1.0 (0.9, 1.1) |
| 25–29 | 120.8 | Ref | 213.7 | Ref | 53.2 | Ref | 74.9 | Ref |
| 30–34 | 121.8 | 1.1 (1.0, 1.1) | 238.5 | 1.1 (1.0, 1.2) | 55.8 | 1.1 (1.0, 1.2) | 98.9 | 1.3 (1.2, 1.5) |
| 35–39 | 147.1 | 1.3 (1.2, 1.4) | 301.0 | 1.3 (1.2, 1.4) | 71.6 | 1.4 (1.3, 1.6) | 134.3 | 1.7 (1.5, 1.9) |
| >=40 | 218.1 | 1.8 (1.6, 2.1) | 397.6 | 1.6 (1.4, 1.8) | 115.4 | 2.3 (1.9, 2.8) | 199.8 | 2.3 (1.9, 2.8) |
| Marital status | ||||||||
| Married | 124.2 | Ref | 215.4 | Ref | 55.8 | Ref | 95.2 | Ref |
| Unmarried | 156.1 | 1.0 (1.0, 1.1) | 240.4 | 1.1 (1, 1.2) | 60.9 | 1.0 (0.9, 1.1) | 87.0 | 1.0 (0.9, 1.1) |
| Insurance payor | ||||||||
| Private | 120.9 | Ref | 203.3 | Ref | 55.2 | Ref | 86.8 | Ref |
| Medicaid | 155.8 | 1.2 (1.1, 1.3) | 242.6 | 1.1 (1.1, 1.2) | 60.1 | 1.1 (0.9, 1.2) | 85.8 | 1.1 (1.0, 1.2) |
| Medicare | 156.6 | 1.2 (1.0, 1.4) | 409.7 | 1.7 (1.5, 2.0) | 80.8 | 1.4 (1.0, 1.7) | 227.4 | 2.4 (1.9, 2.9) |
| Self pay | 152.2 | 1.1 (0.8, 1.4) | 343.1 | 1.4 (1.2, 1.7) | 38.9 | 0.6 (0.4, 1.0) | 139.4 | 1.5 (1.1, 1.8) |
| Other payors | 117.8 | 1.0 (0.9, 1.1) | 200.7 | 1.0 (0.9, 1.1) | 59.1 | 1.1 (0.9, 1.2) | 95.1 | 1.2 (1.0, 1.4) |
| Education | ||||||||
| Less than high school | 166.3 | 1.3 (1.0, 1.6) | 272.4 | 1.2 (1.0, 1.5) | 61.4 | 1.2 (0.8, 1.8) | 91.6 | 1.1 (0.8, 1.6) |
| Some high school | 183.1 | 1.4 (1.2, 1.5) | 268.6 | 1.2 (1.1, 1.3) | 62.0 | 1.2 (1.0, 1.4) | 90.8 | 1.1 (1.0, 1.3) |
| High school degree/GED | 143.9 | 1.1 (1.1, 1.2) | 248.3 | 1.2 (1.1, 1.2) | 60.1 | 1.1 (1.0, 1.3) | 91.1 | 1.2 (1.1, 1.3) |
| Some college or higher | 120.1 | Ref | 209.0 | Ref | 54.4 | Ref | 84.8 | Ref |
| Tobacco use during pregnancy | ||||||||
| No | 132.1 | Ref | 232.0 | Ref | 56.8 | Ref | 88.8 | Ref |
| Yes | 143.1 | 0.9 (0.8, 1.0) | 278.3 | 1.0 (0.9, 1.1) | 60.2 | 1.0 (0.9, 1.2) | 94.3 | 0.9 (0.7, 1.1) |
| NCHS rural-urban | ||||||||
| Large central metro | 117.3 | Ref | 288.1 | Ref | 43.0 | Ref | 96.7 | Ref |
| Large fringe metro | 134.3 | 1.1 (1.0, 1.3) | 235.7 | 0.9 (0.8, 0.9) | 55.0 | 1.4 (1.2, 1.7) | 88.1 | 0.9 (0.8, 1.0) |
| Medium metro | 101.8 | 0.8 (0.7, 0.9) | 169.7 | 0.6 (0.5, 0.6) | 59.6 | 1.4 (1.1, 1.8) | 79.3 | 0.9 (0.7, 1.0) |
| Small metro | 119.7 | 1.0 (0.9, 1.1) | 212.5 | 0.7 (0.7, 0.8) | 51.1 | 1.3 (1.0, 1.6) | 88.9 | 0.9 (0.8, 1.1) |
| Micropolitan | 165.4 | 1.3 (1.2, 1.5) | 259.8 | 0.9 (0.8, 0.9) | 72.5 | 1.8 (1.5, 2.3) | 94.8 | 1.0 (0.9, 1.2) |
| Noncore | 160.2 | 1.3 (1.1, 1.5) | 258.9 | 0.9 (0.8, 1.0) | 66.2 | 1.7 (1.4, 2.2) | 99.3 | 1.1 (0.9, 1.3) |
| Plurality | ||||||||
| Single | 127.4 | Ref | 224.2 | Ref | 55.5 | Ref | 87.1 | Ref |
| Multiple | 465.5 | 3.5 (3.1, 3.8) | 738.3 | 3 (2.7, 3.3) | 160.0 | 2.6 (2.1, 3.0) | 207.1 | 2.0 (1.7, 2.4) |
| Parity | ||||||||
| nulliparity | 149.4 | Ref | 222.9 | Ref | 62.1 | Ref | 97.5 | Ref |
| low multiparity (1–3) | 117.2 | 0.8 (0.7, 0.8) | 225.3 | 1.0 (1.0, 1.1) | 51.9 | 0.8 (0.7, 0.8) | 79.7 | 0.7 (0.7, 0.8) |
| grand multipara (4+) | 206.1 | 1.0 (0.9, 1.2) | 364.0 | 1.3 (1.2, 1.4) | 88.6 | 1.0 (0.8, 1.2) | 120.2 | 0.9 (0.7, 1.0) |
| Prenatal care | ||||||||
| Prenatal Care | 129.4 | Ref | 217.5 | Ref | 54.8 | Ref | 80.6 | Ref |
| Late or no prenatal care | 149.3 | 1.1 (0.9, 1.2) | 269.4 | 1.1 (1.1, 1.2) | 57.0 | 1.0 (0.8, 1.3) | 90.8 | 1.1 (1.0, 1.3) |
| Pre-pregnancy hypertension | ||||||||
| No | 132.1 | Ref | 227.1 | Ref | 56.3 | Ref | 84.3 | Ref |
| Yes | 214.5 | 1.5 (1.3, 1.8) | 461.4 | 1.9 (1.8, 2.1) | 113.3 | 1.8 (1.4, 2.3) | 244.6 | 2.7 (2.3, 3.1) |
| Gestational hypertension | ||||||||
| No | 121.5 | Ref | 210.8 | Ref | 50.4 | Ref | 75.2 | Ref |
| Yes | 322.4 | 2.4 (2.2, 2.6) | 578.9 | 2.6 (2.5, 2.8) | 164.8 | 3.0 (2.7, 3.3) | 293.9 | 3.7 (3.3, 4) |
| Gestational diabetes | ||||||||
| No | 132.0 | Ref | 232.2 | Ref | 56.4 | Ref | 88.1 | Ref |
| Yes | 165.0 | 1.0 (0.9, 1.2) | 281.3 | 1.0 (0.9, 1.1) | 75.1 | 1.0 (0.8, 1.2) | 114.6 | 0.9 (0.8, 1.1) |
SMM rates are events per 10,000 deliveries.
SMM indicates severe maternal morbidity.
FIGURE 1.
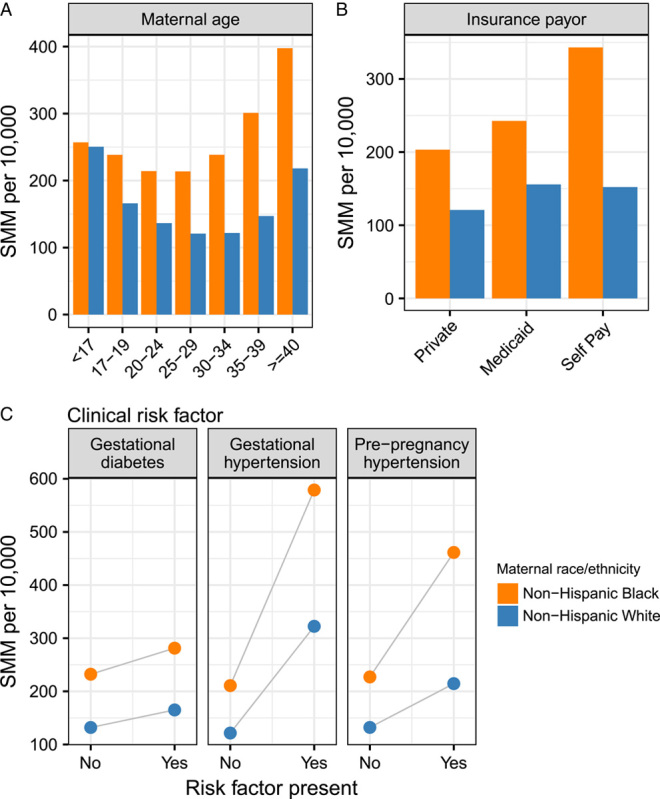
Severe maternal mortality rates per 10,000 deliveries for non-Hispanic Black and non-Hispanic White women in Georgia, 2009–2020 stratified by selected demographic and clinical risk factors, using case definition including blood transfusion. A, Maternal age; B, Delivery insurance payor; C, clinical risk factors, including gestational diabetes, gestational hypertension, and pre-pregnancy hypertension. SMM indicates severe maternal morbidity
In race-stratified multiply adjusted models (Table 2), several factors remained meaningfully and significantly associated with SMM. Using the SMM case definition without blood transfusion, non-Hispanic White and Black pregnant persons 40 years of age and older experienced similarly elevated risk (eg, RR of 2.3, 95% CI 1.9–2.8 for persons 40 or older), but there were racial differences in the magnitude of association for other factors. For example, the risk of SMM was lowest in large central metropolitan counties (eg, those containing and immediately surrounding Atlanta) for non-Hispanic White persons and was higher in every other kind of county including medium and small metropolitan, micropolitan and rural non-core counties. However, SMM risk was relatively homogenous—albeit higher overall—for non-Hispanic Black persons regardless of urbanicity or rurality. Pre-pregnancy and gestational hypertension were strong predictors of SMM risk for all pregnant persons, but the magnitude of association was greater for non-Hispanic Black as compared with White persons.
The annual SMM rate varied over time for both SMM definitions with and without blood transfusion, and breaks in the trend line are apparent with the adoption of the ICD-10-CM codes in 2015 (Fig. 2, Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/MLR/C588). Since 2015, SMM rates, including blood transfusion, have been increasing, but there was only a modest increase for SMM without blood transfusion between 2015 and 2019 and evidence of a decline in 2020.
FIGURE 2.
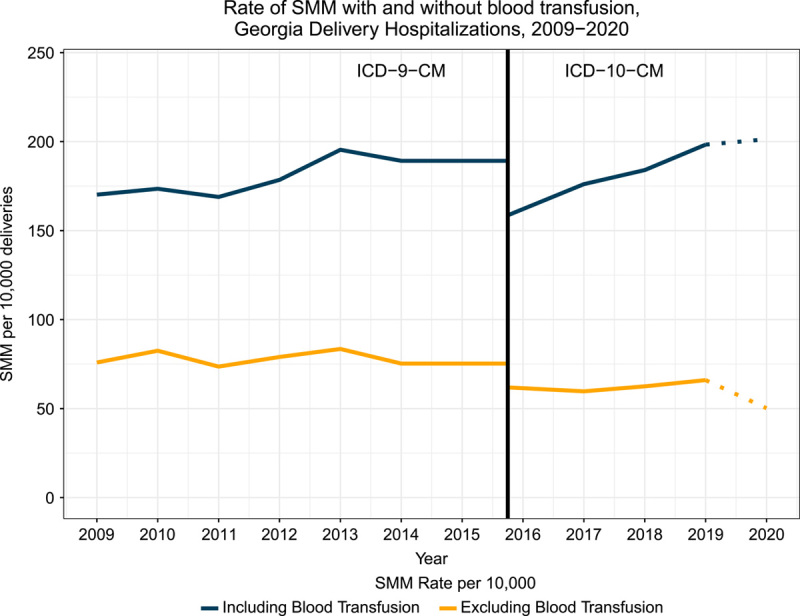
Annual SMM events per 10,000 deliveries among Georgia resident women ages 12–55 between 2009–2020, using case definition including and excluding blood transfusions. The break between the use of ICD-9-CM and ICD-10-CM codes reflects the change in CDC/AIM codes defining SMM events across ICD version. The dotted lines from 2019–2020 reflect the overlay of the COVID-19 pandemic. SMM indicates severe maternal morbidity.
The 5 most frequent SMM indicators for Georgia resident women during the delivery hospitalization were: (1) Blood transfusion (n=14,247; SMM rate 121.5 per 10,000 delivery hospitalizations); (2) Disseminated intravascular coagulation (n=3,124; SMM rate 26.6 per 10,000); (3) Hysterectomy (n=1,087; SMM rate 9.3 per 10,000); (4) Adult respiratory distress syndrome (n=1,070; SMM rate 9.1 per 10,000); and (5) Acute renal failure (n=1,026; SMM rate 8.7 per 10,000) (Supplemental Table 1, Supplemental Digital Content 3, http://links.lww.com/MLR/C590). Eclampsia, pulmonary edema, shock, and sepsis were also relatively frequent indicators of SMM (Supplemental Table 1, Supplemental Digital Content 3, http://links.lww.com/MLR/C590).
Persons that delivered live-born infants at a gestational age <37 weeks were at higher risk of SMM compared with those that delivered a term infant, with a progressive increase in risk for those with a late preterm delivery, through a very preterm delivery (Table 3). Similarly, those delivering infants with birth weights <3000 grams or >4000 grams were at elevated risk of SMM compared with those delivering infants between 3000 and 4000 grams.
TABLE 3.
Frequency of Neonatal Outcomes for Severe Maternal Morbidity (SMM) Events With and Without Blood Transfusion During the Delivery Hospitalization, Among Georgia Resident Women Ages 12–55 Between 2009–2020
| Including blood transfusion | Excluding blood transfusion | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Delivery hospitalizations (%) | SMM events (%) | SMM rate | Crude RR (95% CI) | SMM events (%) | SMM rate | Crude RR (95% CI) |
| Gestational age in weeks | |||||||
| Extremely preterm (<28) | 9,944 (1) | 724 (3) | 728.1 | 5.8(5.4, 6.2) | 724 (3) | 728.1 | 8.9(8.0, 9.9) |
| Very preterm (28-<32) | 12,165 (1) | 959 (5) | 788.3 | 6.3 (5.9, 6.7) | 959 (5) | 788.3 | 10.5 (9.5, 11.5) |
| Moderate preterm (32-<34) | 13,786 (1) | 931 (4) | 675.3 | 5.4 (5.0, 5.7) | 931 (4) | 675.3 | 8.9 (8.1, 9.8) |
| Late preterm (34-<37) | 86,591 (7) | 3,173 (15) | 366.4 | 2.9 (2.8, 3.0) | 3,173 (15) | 366.4 | 4.2 (4.0, 4.5) |
| Early term (37-<39) | 326,784 (28) | 5,714 (28) | 174.9 | 1.4 (1.3, 1.4) | 5,714 (28) | 174.9 | 1.6 (1.5, 1.7) |
| Full term (39-<41) | 671,289 (57) | 8,435 (41) | 125.7 | Ref | 8,435 (41) | 125.7 | Ref |
| Late term (>=41) | 52,193 (4) | 765 (4) | 146.6 | 1.2 (1.1, 1.3) | 765 (4) | 146.6 | 1.0 (0.9, 1.2) |
| Birth weight (grams) | |||||||
| <1500 | 19,889 (2) | 1,545 (7) | 776.8 | 5.6 (5.2, 5.9) | 1,545 (7) | 776.8 | 9.5 (8.7, 10.4) |
| 1500–1999 | 19,200 (2) | 1,176 (6) | 612.5 | 4.4 (4.1, 4.7) | 1,176 (6) | 612.5 | 7.2 (6.5, 7.9) |
| 2000–2499 | 65,536 (6) | 2,159 (10) | 329.4 | 2.4 (2.2, 2.5) | 2,159 (10) | 329.4 | 3.4 (3.2, 3.7) |
| 2500–2999 | 239,586 (20) | 4,196 (20) | 175.1 | 1.3 (1.2, 1.3) | 4,196 (20) | 175.1 | 1.6 (1.4, 1.7) |
| 3000–3499 | 464,795 (40) | 6,154 (30) | 132.4 | 0.9 (0.9, 1.0) | 6,154 (30) | 132.4 | 1.0 (1.0, 1.1) |
| 3500–3999 | 286,310 (24) | 4,006 (19) | 139.9 | Ref | 4,006 (19) | 139.9 | Ref |
| 4000–4499 | 66,301 (6) | 1,165 (6) | 175.7 | 1.3 (1.2, 1.3) | 1,165 (6) | 175.7 | 1.2 (1.0, 1.3) |
| >=4500 | 10,272 (1) | 228 (1) | 222.0 | 1.6 (1.4, 1.8) | 228 (1) | 222.0 | 1.4 (1.1, 1.8) |
| Fetal/neonatal death | |||||||
| Fetal death | 5,337 (0) | 470 (2) | 880.6 | 5.1 (4.6, 5.6) | 470 (2) | 880.6 | 7.7 (6.8, 8.7) |
| Live birth | 1,167,415 (100) | 20,231 (98) | 173.3 | Ref | 20,231 (98) | 173.3 | Ref |
Patterns of maternal and neonatal factors associated with SMM were consistent with those summarized above when we allowed SMM to be defined at either the delivery hospitalization or any post-delivery rehospitalization within 42 days of delivery (Supplemental Table 3-4, Supplemental Digital Content 2, http://links.lww.com/MLR/C589, Supplemental Digital Content 4, http://links.lww.com/MLR/C591). We considered that results in 2020 might have differed due to COVID-19 pandemic-related changes in health care and surveillance. However, results were not meaningfully different when pooled analysis excluded deliveries in 2020 (data not shown).
DISCUSSION
During the delivery hospitalization, Georgia resident women experienced 176.5 and 69.4 SMM events per 10,000 deliveries between 2009 and 2020 for definitions including and excluding (respectively) blood transfusions. From 2015, when the ICD10 code adoption began, through 2019, SMM rates have increased in Georgia for both SMM definitions that included and excluded blood transfusion, although the trend is more pronounced for the definition including blood transfusions. The appearance of a slight decline in SMM excluding blood transfusion in 2020 warrants further investigation, given other anomalous perinatal surveillance trends in 2020 likely related to the COVID-19 pandemic and response.26
The Georgia SMM rate during our study period was substantially higher than the US SMM rate. For example, comparing estimates in 2014 (the most recent national estimate available from CDC27), the US rate of SMM, including blood transfusion, was 144 per 10,000 deliveries compared with the Georgia 2014 rate of 189.2 (95% CI: 181.0, 197.3) per 10,000. The national rate without blood transfusions was 35 per 10,000 as compared with Georgia’s 2014 rate of 75.3 (95% CI: 70.1, 80.5) per 10,000. The upward trend of Georgia’s annual SMM rate parallels the upward trend of the overall US SMM rate over a similar timeframe.22,27 The 5 most common indicators of SMM in Georgia during the study timeframe were blood transfusion, disseminated intravascular coagulation, hysterectomy, adult respiratory distress syndrome, and acute renal failure. These are similar to the national leading indicators of SMM.27
As is the case for many health outcomes, in this study, SMM risk was associated with extremes of age, membership in a minoritized racial or ethnic group, indicators of lower socioeconomic status, and the presence of chronic conditions. Morbidity among these peripartum persons was also understandably associated with antepartum complications and poor feto-infant outcomes. The patterns have important public health and health care implications. For example, Georgia has a large and unacceptable excess risk for SMM among pregnant persons of color, particularly Black as compared with non-Hispanic White persons. But it is also notable that the Black-White racial disparity in SMM in Georgia (eg, unadjusted RR of 1.6) is actually smaller in magnitude than reports of corresponding disparities in other states (eg, unadjusted racial disparities ranging from RR 1.8–2.1).16,28,29
Direct comparisons of the current study to others are made difficult by differing study time periods and, in the case of adjusted models, different covariates in each study. However, 1 possible explanation for apparently smaller relative racial disparities in SMM in Georgia is that all Georgia pregnant people, including non-Hispanic White, face somewhat higher risk as compared with those delivering in other states reported in the literature. For example, Leonard, et al,29 report that non-Hispanic White persons in California had an SMM rate of 84 per 10,000 overall and 42 per 10,000 excluding transfusion. In contrast, non-Hispanic White pregnant persons in Georgia experienced 133 (including transfusions) and 57 (excluding transfusions) SMM events per 10,000 delivery hospitalizations. These differences emphasize the importance of conducting a regionalized study of the patterns of maternal health risk overall and within specific marginalized groups, including communities of color, where exposure to structural and social determinants of health may differentially shape experiences before, during and after pregnancy.
Excess risk of SMM in Georgia related to rurality, insurance status, comorbid chronic conditions, and poor socioeconomic status each speak to the importance of social determinants of health and health care access in shaping maternal health outcomes, as documented in a recent systematic review.30 Medicaid expansion, which decreases the uninsured rate for women of reproductive age before and between pregnancies, is seen as a key strategy for reducing maternal morbidity and mortality rates.31,32 The expansion of Medicaid, which improves access to health care services before and between pregnancies, has been associated with increased receipt of preconception health counseling around health risks33 and with a significantly reduced rate of SMM.34 Rates of maternal mortality from 2006 to 2017 are also noted to be lower in expansion versus non-expansion states.35 Analysis of Medicaid claims data across multiple states from 2010 to 2012 supports that specific domains of preconception care, including receipt of contraceptive services and pregnancy testing services, significantly decrease the odds of SMM; in restricting the study population to Medicaid-enrolled women with chronic health conditions, both the receipt of contraceptive services and routine physical examinations in the year before conception significantly decreased the odds of SMM.36 Georgia has not expanded Medicaid under the Affordable Care Act, but in 2021 did extend postpartum Medicaid coverage from 2 to 6 months; while this extension of coverage during the postpartum period is critical for addressing health needs in the “fourth trimester,”37 it does little to increase access to primary and preventive health care before conception, especially for women without dependent children.31 Georgia did implement a Section 1115 Medicaid Demonstration Family Planning Waiver in 2011, which expanded the provision of family planning services to uninsured 18 to 44 year old women citizens of Georgia with a household income at or below 211% of the federal poverty level who are not otherwise eligible for Medicaid or the Children’s Health Insurance Program. Implementation of the Georgia Family Planning Demonstration Waiver contributed to an increase in the use of higher effectiveness contraceptive methods and the increased use of preventive screenings; however, a substantial proportion of low-income women targeted by the waiver remain unserved by the publicly-funded family planning services in the state.38
Strengths and Limitations
Strengths of this study include its use of a large, population-based dataset of Georgia resident pregnant persons delivering in Georgia between 2009 and 2020. However, because the determination of the occurrence of SMM requires ICD diagnosis code data, such as that found in the Georgia Discharge Data System, a requirement for inclusion in the study cohort was that a Georgia resident has a delivery that is linked to a hospital discharge record, thus excluding deliveries at home or in birth centers. However, this is only about 1% of live births 39 and likely will tend to be lower-risk pregnancies on average. Strength of the study was the enrichment of the dataset variables through the linkage between hospital discharges and vital records such as live birth and fetal death certificates. However, restricting to records that are successfully linked can lead to potential biases if the probability of accurate linkage is differential with respect to important covariates. Individuals who were excluded due to the inability to link were more likely to be on public insurance (58% vs. 50%) and more likely to be of minority race and ethnicity (66% vs. 51%). Administrative hospital discharge databases are created and used for billing rather than patient care and are subject to errors of omission and commission by medical coders. Further analysis is needed to explore the significance of these potential biases and errors and their effect on the interpretation and overall validity of findings related to SMM. In addition, there is evidence that reliance on ICD codes for blood transfusion, which do not distinguish the number of units, likely captures deliveries that were not actually clinically severe.40 While we rely on the CDC/AIM indicator list, it may be that future definitions or more formal development of dedicated SMM surveillance systems will refine case ascertainment by, for example, combining information on disseminated intravascular coagulation, intensive care unit admission, and transfusion.
CONCLUSION
The upward trend of SMM rates in the United States is a major public health concern. This investigation was 1 of the most comprehensive evaluations of the burden, trends, and disparities of SMM in a Southeastern state with among the highest maternal mortality rate in the nation. Despite the limitations described above, it lays the groundwork for continued assessment that can ultimately inform clinical, programmatic, and policy interventions. The study provides a guiding framework on the value of enhanced surveillance through statewide data linkages for other southern states to understand the burden of SMM in high-risk populations. Future studies should move beyond state-level assessment and examine geographic differences in SMM rates among sub-state regions to inform place-based changes in quality care and health policy targets. Advancing research on a standardized surveillance approach is critical to elucidate health system failures and intervention priorities to prevent a pregnant person’s progression along the continuum of severity. Ongoing surveillance of both maternal mortality (through the Georgia Maternal Mortality Review Committee) and SMM outcomes may improve the identification of issues contributing to maternal progression along the peripartum morbidity continuum. Ultimately, the goals of this study and its corollaries are to enhance the allocation of Georgia’s limited public health and obstetric resources and to improve upon our poor maternal and infant health outcomes. Georgia has a long road ahead to meet these aims, but the state has taken several steps on the path to improvement in maternal and infant health.
Supplementary Material
Footnotes
This project was supported in part by the American College of Obstetrics and Gynecology/Warren H. Pearse Women’s Health Policy Research Award (Dr. Zertuche); by the Health Resources and Service Administration (HRSA) Maternal and Child Health Bureau grant T76MC28446 to the Emory Maternal and Child Health Center of Excellence; and by National Institute of Health/National Institute on Minority Health and Health Disparities R01MD016031.
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.lww-medicalcare.com.
Contributor Information
Michael R. Kramer, Email: mkram02@emory.edu.
Katie Labgold, Email: catherine.anne.labgold@emory.edu.
Adrienne D. Zertuche, Email: zertuchemd@gmail.com.
Jennifer D. Runkle, Email: jrrunkle@ncsu.edu.
Michael Bryan, Email: michael.bryan@dph.ga.gov.
Gordon R. Freymann, Email: gordon.freymann@dph.ga.gov.
David Austin, Email: dpaustin@dhr.state.ga.us.
E. Kathleen Adams, Email: eadam01@emory.edu.
Anne L. Dunlop, Email: amlang@emory.edu.
REFERENCES
- 1.Walker D, Boling K. Black maternal mortality in the media: How journalists cover a deadly racial disparity. Journalism. Published online January 3, 2022:14648849211063360. doi: 10.1177/14648849211063361 [DOI]
- 2.U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Improve Maternal Health.; 2020:71. Accessed February 8, 2022. https://www.hhs.gov/sites/default/files/call-to-action-maternal-health.pdf [DOI] [PubMed]
- 3.Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: Factors associated with severity. Am J Obstet Gynecol. 2004;191:939–944. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System | Maternal and Infant Health | CDC. Published November 25, 2020. Accessed July 27, 2022. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm
- 5.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Washington DC: Healthy People; 2020. https://www.healthypeople.gov/2020 [Google Scholar]
- 6.Tikkanen R Gunja M FitzGeral M, et al. . Maternal Mortality and Maternity Care in the United States Compared to 10 Other Developed Countries. doi: 10.26099/411v-9255 [DOI]
- 7.Admon LK, Winkelman TNA, Zivin K, et al. Racial and ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012–2015. Obstet Gynecol. 2018;132:1158–1166. [DOI] [PubMed] [Google Scholar]
- 8.Geller SE, Cox SM, Callaghan WM, et al. Morbidity and mortality in pregnancy: laying the groundwork for safe motherhood. Womens Health Issues Off Publ Jacobs Inst Womens Health. 2006;16:176–188. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–1036. [DOI] [PubMed] [Google Scholar]
- 10.Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003. Am J Obstet Gynecol. 2008;199:133.e1–8. [DOI] [PubMed] [Google Scholar]
- 11.Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12:469–477. [DOI] [PubMed] [Google Scholar]
- 12.Leonard SA, Main EK, Carmichael SL. The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy Childbirth. 2019;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Snowden JM, Lyell DJ, et al. Interpregnancy interval and subsequent severe maternal morbidity: a 16-year population-based study from California. Am J Epidemiol. 2021;190:1034–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Main EK, Leonard SA, Menard MK. Association of maternal comorbidity with severe maternal morbidity: a cohort study of California mothers delivering between 1997 and 2014. Ann Intern Med. 2020;173:S11–S18. [DOI] [PubMed] [Google Scholar]
- 15.El Ayadi AM, Baer RJ, Gay C, et al. Risk factors for dual burden of severe maternal morbidity and preterm birth by insurance type in California. Matern Child Health J. 2022;26:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu SY, Fiorentini C, Bailey Z, et al. Structural racism and severe maternal morbidity in New York State. Clin Med Insights Womens Health. 2019;12:1179562X19854778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell EA, Egorova NN, Balbierz A, et al. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016;215:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell EA, Egorova N, Balbierz A, et al. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol. 2016;214:122.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcox W, Johansson EW, Liu SY, et al. Determinants of severe maternal morbidity and its racial/ethnic disparities in New York City, 2008–2012. Matern Child Health J. 2019;23:346–355. [DOI] [PubMed] [Google Scholar]
- 20.Salahuddin M, Mandell DJ, Lakey DL, et al. Maternal comorbidity index and severe maternal morbidity during delivery hospitalizations in Texas, 2011-2014. Birth. 2020;47:89–97. [DOI] [PubMed] [Google Scholar]
- 21.Creanga AA, Bateman BT, Kuklina EV, et al. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008-2010. Am J Obstet Gynecol. 2014;210:435.e1–435.e8. [DOI] [PubMed] [Google Scholar]
- 22.Hirai AH, Owens PL, Reid LD, et al. Trends in severe maternal morbidity in the US across the transition to ICD-10-CM/PCS From 2012-2019. JAMA Netw Open. 2022;5:e2222966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgia Department of Public Health. Maternal Mortality Fact Sheet: 2015-2017 Data. 2022. Accessed July 27, 2022. https://dph.georgia.gov/document/document/maternal-mortality-factsheet-2015-2017-data/download
- 24.Alliance for Innovation on Maternal Health. Alliance for Innovation of Maternal Health (AIM) SMM Codes v08-09-2021. Published online 2021. Accessed December 6, 2021. https://safehealthcareforeverywoman.org/aim/resources/aim-data-resources/.
- 25.R Core Team. R: A language and envrionment for statistical computing. Published online 2021. Accessed July 2021. https://www.R-project.org/
- 26.Hamilton B, Martin J, Osterman M. Births: Provisional Data for 2020. Hyattsville: National Center for Health Statistics; 2021. doi: 10.15620/cdc:104993. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Rates in Severe Morbidity Indicators per 10,000 Delivery Hospitalizalization | Maternal Infant Health | Reproductive Health | CDC. Published February 8, 2021. Accessed November 23, 2021. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/rates-severe-morbidity-indicator.htm
- 28.Conrey EJ, Manning SE, Shellhaas C, et al. Severe maternal morbidity, a tale of 2 States using data for action—Ohio and Massachusetts. Matern Child Health J. 2019;23:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard SA, Main EK, Scott KA, et al. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol. 2019;33:30–36. 10.1016/j.annepidem.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang E, Glazer KB, Howell EA, et al. Social determinants of pregnancy-related mortality and morbidity in the United States: A systematic review. Obstet Gynecol. 2020;00:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston EM, Strahan AE, Joski P, et al. Impacts of the affordable care act’s medicaid expansion on women of reproductive age: differences by parental status and state policies. Womens Health Issues. 2018;28:122–129. [DOI] [PubMed] [Google Scholar]
- 32.Johnston EM, McMorrow S, Alvarez Caraveo C, et al. Post-ACA, More than one-third of women with prenatal medicaid remained uninsured before or after pregnancy: study examines insurance coverage and access to care before, during, and after pregnancy for women with prenatal Medicaid coverage. Health Aff. 2021;40:571–578. [DOI] [PubMed] [Google Scholar]
- 33.Myerson R, Crawford S, Wherry LR. Medicaid expansion increased preconception health counseling, folic acid intake, and postpartum contraception: study examines the impact of ACA Medicaid expansion on health behaviors including birth control use and pregnancy intention, and receipt of preconception health services. Health Aff. 2020;39:1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guglielminotti J, Landau R, Li G. The 2014 New York State medicaid expansion and severe maternal morbidity during delivery hospitalizations. Anesth Analg. 2021;133:340–348. [DOI] [PubMed] [Google Scholar]
- 35.Eliason EL. Adoption of medicaid expansion is associated with lower maternal mortality. Womens Health Issues. 2020;30:147–152. [DOI] [PubMed] [Google Scholar]
- 36.Dude AM, Schueler K, Schumm LP, et al. Preconception care and severe maternal morbidity in the United States. Am J Obstet Gynecol MFM. 2022;4:100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tully KP, Stuebe AM, Verbiest SB. The fourth trimester: a critical transition period with unmet maternal health needs. Am J Obstet Gynecol. 2017;217:37–41. [DOI] [PubMed] [Google Scholar]
- 38.Dunlop AL, Adams EK, Hawley J, et al. Georgia’s medicaid family planning waiver: working together with Title X to enhance access to and use of contraceptive and preventive health services. Womens Health Issues. 2016;26:602–611. [DOI] [PubMed] [Google Scholar]
- 39.Gregory ECW. Changes in Home Births by Race and Hispanic Origin and State of Residence of Mother: United States, 2018–2019 and 2019–2020. National Vital Statistics Reports. 2021;70:10. [PubMed]
- 40.Friedman AM, Oberhardt M, Sheen JJ, et al. Measurement of hemorrhage-related severe maternal morbidity with billing versus electronic medical record data. J Matern Fetal Neonatal Med. 2022;35:2234–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]


