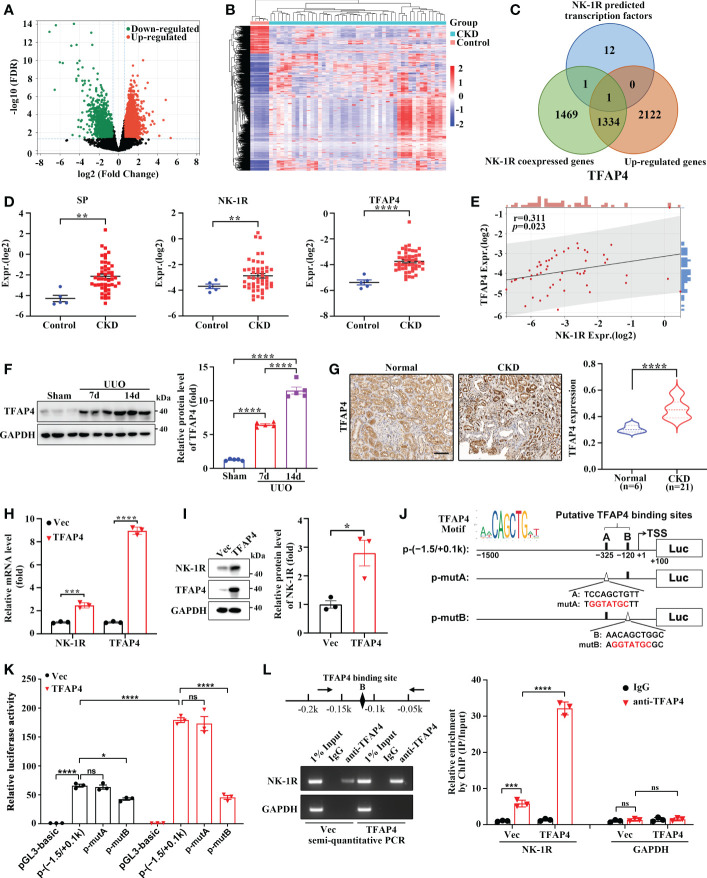
Figure 6.
TFAP4 is dysregulated in fibrotic kidneys and transcriptionally activates NK-1R expression. (A, B) The volcano plot (A) and heatmap (B) of DEGs between CKD and non-CKD patients from GSE66494 with the threshold of FDR < 0.05 and |log2 (Fold Change)| > = 1. (C) Venn diagram of ConTra v3-predicted NK-1R transcription factors overlapping NK-1R co-expressed genes and up-regulated genes in GSE66494. (D) The mRNA levels of renal NK-1R, SP, and TFAP4 increased in CKD patients based on GSE66494. (E) Correlation between the expression of NK-1R and TFAP4 in GSE66494. (F, G) Western blotting (F) and immunochemistry staining (G) showed the up-regulated expression of renal TFAP4 protein in UUO mice and CKD patients, respectively. (H, I) Real-time quantitative PCR (H) and Western blotting (I) showed that the stable overexpression of TFAP4 increased NK-1R mRNA and protein levels in HK-2 cells. (J, K) Overexpression of TFAP4 enhanced the activity of the NK-1R promoter, whereas the mutation of the potential TFAP4 binding site B but not A reduced its activity. Schematic diagram for firefly luciferase reporter plasmids containing -1.5 to +0.1 k region of NK-1R is shown. Potential TFAP4 binding sites (BS) in the NK-1R promoter are depicted as a close rectangle A/B, and mutant TFAP4 BS are depicted as an open triangle A/B. (L) ChIP assay showed that TFAP4 directly interacted with the NK-1R promoter in vivo. The antibody-precipitated DNA was amplified by semi-quantitative PCR for 30 cycles (left) and real-time quantitative PCR (right). The promoter of GAPDH was used as a negative control. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, not significant.