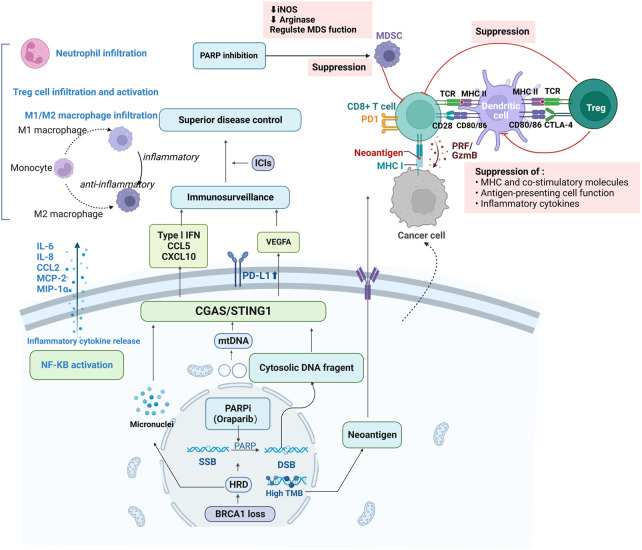
FIGURE 4.
Synergy of immune checkpoint inhibitors with PARPi and tumor suppressive immune microenvironment. PARPis induces double-strand breaks in HRD cells, generating cytoplasmic dsDNA fragments, micronuclei and mtDNA, which trigger the activation of the STING pathway by binding to cyclic GMP-AMP synthase (cGAS); on the one hand, it upregulates the secretion of type I interferon, CCL5, CXCL10 and VEGFA, promoting immune escape. On the other hand, low levels of DNA damage stimulate infiltration of suppressive immune cells, such as myeloid-derived suppressor cells (MDSC) or tumor-associated macrophage (TAM), which leads to the release of free radicals and triggers further DNA damage. Antigen-presenting cells, including tumor-associated dendritic cells, are recruited and activated to drive STING-dependent type I IFN signaling. Increased expression of T cell-associated chemokines activates CD4+ and CD8+ T lymphocytes in tumor and bone marrow-derived cells, increasing infiltration of cytotoxic T-cell in the microenvironment and promoting reprogramming of immune cells to phenotypes with antitumor activity. PARPis also activates PD-L1 transcription, PD-1/PD-L1 blockade and enhances antitumor immunity; activation of the κB pathway and release of various cytokines inhibit epithelial-mesenchymal transition (EMT), thereby increasing immune cell infiltration at tumor sites.