Abstract
Objective
Sacroiliac joint (SIJ) pain is a common cause of chronic low back pain. Full-endoscopic rhizotomy of lateral branches of dorsal rami innervating SIJ is a potential option for patients’ refractory to medical treatment. The full-endoscopic rhizotomy is sometimes challenging under fluoroscopic guidance. This study is to evaluate the effectiveness of the navigation-assisted full-endoscopic rhizotomy for SIJ pain.
Methods
The study was a retrospective match-paired study that enrolled consecutive patients undergoing navigation-assisted full-endoscopic rhizotomy for SIJ pain. The patient demographics, clinical outcomes, and operative parameters of endoscopic rhizotomy were compared with conventional cooled radiofrequency ablation (RFA) treatment.
Results
The study enrolled 72 patients, including 36 patients in the endoscopic group. Thirty-six patients in the cooled RFA group were matched by age as the control. The follow-up time was at least 1 year. Patient characteristics were similar between the groups. The navigation-assisted endoscopic rhizotomy operation time was significantly longer than the cooled RFA. The visual analogue scale (VAS) for pain and Oswestry Disability Index (ODI) significantly decreased after each treatment. However, the between-group comparison revealed that the VAS and ODI of the patients after endoscopic rhizotomy were significantly lower than those after the cooled RFA group. There were no postoperative complications in the study.
Conclusion
Navigation-assisted full-endoscopic rhizotomy is an alternative to SIJ pain treatment. Integrating intraoperative navigation can ensure accurate full-endoscopic rhizotomy to provide better durability of pain relief than the cooled RFA.
Keywords: Endoscopic rhizotomy, Navigation, Sacroiliac joint, Radiofrequency ablation
INTRODUCTION
Sacroiliac joint (SIJ) pain is a common cause of chronic low back pain (CLBP). In about 25% of patients with CLBP, SIJ is the pain generator [1]. The SIJ is a diarthrosis-amphiarthrosis joint between the sacrum and the ilium, which transfers weight from the axial skeleton to the lower extremities. The strong interconnecting ligaments surrounding the SIJ and the tight wedging of the sacrum between hip bones make the SIJ relatively immobile [2]. The SIJ pain may result from capsular and ligamentous tension, hypo- or hypermobility, extraneous compression, or shearing forces [3]. The etiologies of SIJ injury could be traumatic or atraumatic. Because SIJ dysfunction lacks pathognomonic physical examination or radiographic findings, it is often overlooked and subsequently undertreated. The diagnosis of SIJ pain requires a combination of different modalities, including a comprehensive history and physical examinations [1,4-6]. The provocative maneuvers and diagnostic injection are helpful to confirm the diagnosis [7,8]. The first-line treatment can be analgesics, exercise programs, physical therapy, or chiropractic manipulation.
The interventional treatments are indicated for SIJ pain refractory to conservative treatment. The common interventions for SIJ pain include extra-articular or intra-articular injections and radiofrequency ablation (RFA) of lateral branch nerves innervating the SIJ [6,9,10]. RFA is considered technically demanding, mainly due to variable patterns of SI joint innervation between patients [4,11]. Besides, the efficacy of RFA for SIJ pain was short-lived in the previous study indicating the pain relapse due to nerve regrowth after lesioning rather than cutting nerve off [12]. Recurrence may occur and require repeated treatment during the long-term follow-up period.
Recently, endoscopic RFA of the SIJ complex has been reported to treat SIJ pain successfully. Under endoscopic visualization, the posterior sacroiliac ligament and its overlying soft tissue were ablated using a bipolar radiofrequency probe through the endoscope’s working channel. Choi et al. [13] used the bipolar radiofrequency to ablate the lateral branches of S1, S2, S3, and the L5 dorsal ramus innervating the posterior capsule of the SIJ. The clinical outcome was favorable in the preliminary study. Sometimes, it is challenging to identify anatomical landmarks such as the dorsal sacral foramen by fluoroscopy, especially when the patient had previous fusion surgery with instrumentation.
The intraoperative computerized stereotactic navigation with computed tomography (CT)-based image modalities has been applied in minimally invasive spine surgery (e.g., O-Arm-Medtronic, Brainlab, and others) [14-16]. The computer-assisted navigation systems can provide reconstructed information in 3 dimensions, and surgeons can immediately interpret surgical anatomy on a navigation screen. The authors have reported an innovative technique of using full-endoscopic rhizotomy of SIJ innervation assisted with a navigation system [17]. However, there is a lack of studies regarding the clinical efficacy comparing the innovative technique with the conventional one. In the current study, the authors reported the case series and comparative analysis with the cooled RFA treatment for SIJ pain.
MATERIALS AND METHODS
1. Patient Enrollment
The retrospective study was approved by the Institutional Review Board of Changhua Christian Hospital (No. 220306). The informed consent from all patients was collected before treatment. All the operations were performed by the same surgeon (first author). The authors collected medical records of consecutive patients who underwent the full-endoscopic rhizotomy of the SIJ pain for CLBP between January 2018 and August 2020. The diagnostic criteria included patients presenting CLBP with or without previous spine surgery that lasts more than 6 months refractory to conservative treatment; the pain was located in the area of the SIJ, approximately 1 to 3 cm inferior to the ipsilateral posterior superior iliac spine; the pain triggers at the inferomedial to the posterior superior iliac spine [18]; physical exam shows more than 3 positive out of 6 provocative tests, including distraction, compression, thigh thrust, Gaenslen test, sacral thrust, and the drop test [19]; double ultrasound guided SIJ injections relieved the pain over 50% temporarily. Radiological images were used to exclude other pain generators such as discogenic back pain or facet joint syndrome [20], and diagnostic blocks were performed for differential diagnosis. Patients with infection, malignancies, or instability were excluded. The age-matched control group was retrospectively collected from the patients undergoing the cooled RFA group in the prospective registry database. The diagnostic and exclusion criteria were the same in both groups, and the follow-up time was at least 1 year.
2. Surgical Procedures: Full-Endoscopic Rhizotomy Assisted With Navigation
The patient was placed in a prone position on a radiolucent table in a hybrid operative room equipped with a 3-dimensional (3D) robotic C-arm system (ARTIS pheno, Siemens Healthineers, Erlangen, Germany). Patients were under local anesthesia during all procedures. After sterile preparation and draping of the surgical site, the reference frame was firmly fixed on the skin with 2 layers of iodine-impregnated incision drapes. The robotic C-arm scanned the patient to obtain computed tomography of the surgical site. Intraoperative virtual images of the SI joints were processed and registered automatically into the image-guided surgery platform (BuzzTM Digital O.R., Brainlab, Munich, Germany).
Matching accuracy was confirmed by placing the navigation pointer on the reference frame. After confirming the matching accuracy, registration of a 5-mm obturator with trackers was done by inserting the corresponding size of the calibrating device. The navigation system helped to determine an entry point at the S1 foramen level. Injection of local anesthesia was done. A stab incision with a No.15 blade was made. Integrate the obturator with a working cannula (an inner diameter of 5.4 mm). The integrated obturator-working channel composite was inserted and docked at the lateral edge of the S1 foramen (Fig. 1). After removing the obturator, a 30° spinal endoscope with a 2.8 working channel and an outer diameter of 5.3 mm (Spinendos GmbH, Munich, Germany) was inserted. The endoscopic procedure was done under continuous saline irrigation. A bipolar coagulator (Vantage Biotech Co., Ltd., Taoyuan, Taiwan) was used for both hemostasis and ablation. The concordant pain response can help the surgeon locate the lateral sacral branch by stimulating the bipolar coagulator tip. The endoscopic micro punch can cut the nerve branch. Further ablation of the nerve stumps and surrounding soft tissues helped enhance the durability of rhizotomy (Fig. 2). The surgeon repeated the “cut-and-ablation” procedure at the lateral margin of the sacral foramen until the absence of triggered pain. After rhizotomies of the lateral sacral branch at the S1 level, the working cannula was shifted cranially and docked at the lateral border of the S1 superior articular process. The cut-and-ablation procedure was repeated for rhizotomy of the L5 dorsal ramus. Finally, the lateral branch rhizotomies at the S2 and S3 levels were conducted surrounding the lateral border of the sacral foramen. The pain relief was confirmed by direct compression of the trigger points [18]. The wound was closed with a single suture.
Fig. 1.
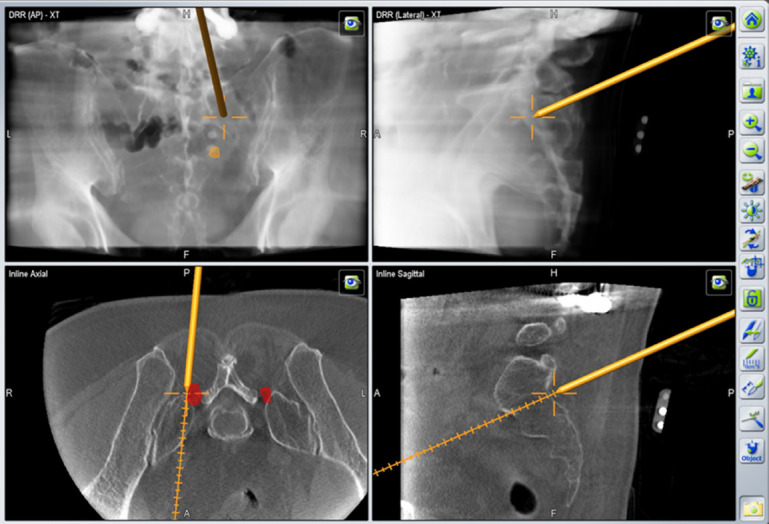
The constructed intraoperative virtual images of the sacroiliac joints.
Fig. 2.
Endoscopic views of lateral branch rhizotomy. (A) The endoscopic visualization of the lateral branch of S1 dorsal ramus. (B) Rhizotomy by the micro punch. (C) Ablating the nerve stumps and the surrounding tissues by the bipolar electrocautery.
3. Surgical Procedures: Cooled RFA
The patient was lying prone on a radiolucent table with a C-arm. Using an anteroposterior fluoroscopic view with tilt cranially (about 20°), the plane of the SI joint, the S1, S2, and S3 sacral foramen were identified. Seven 25-gauge, 3.5-inch Quincke tip needles were inserted toward the lateral base of the S1 superior articular process for the L5 dorsal ramus and 7–10 mm away from the posterior sacral foramen of S1, S2, and S3 [11]. When the tip of the needle contacts the periosteum, needle position was confirmed by intraoperative fluoroscopy. The cooled radiofrequency probe (COOLIEF, Avanos Medical, Inc., Alpharetta, GA, USA) was inserted through the cannulated needles. The L5 dorsal ramus and the lateral sacral branches of S1–3 were ablated at a temperature of 80 degrees for 3 minutes [21]. After removing the needles, the pain relief while pressing the trigger points was the end-point of the intervention.
4. Clinical Assessment
The authors collected patient data prospectively recorded by the clinical research coordinator in the registry database. Operative details such as operation time, blood loss, and complications were included. Visual analogue scale (VAS) score of the back and leg was used to evaluate pain severity. Functional disability was quantified by Oswestry Disability Index (ODI) scores. MacNab criteria were used to group patients according to satisfaction rate. The clinical research coordinator obtained patient-reported outcomes by questionnaire when patients visited the outpatient clinic preoperatively and at 1, 3, 6, and 12 months after the operation.
5. Statistical Analysis
The MedCalc ver. 20.110 (MedCalc Software Ltd., Ostend, Belgium) was the tool for statistical analysis and graphs. After finding out the normal distribution and the variances, the chi-square test, the Mann-Whitney U-test, and the independent t-test was done to compare both groups. Friedman test was done to compare the median values at different times of each group, and p-values below 0.05 were considered significant.
RESULTS
There were 40 consecutive patients undergoing the full-endoscopic rhizotomy between January 2018 and Aug 2020. Four patients did not have complete 1-year follow-ups and were excluded. The control group comprised patients undergoing cooled RF ablation for SIJ pain. By matching age, 36 patients were enrolled in the cooled RF group. As for clinical presentation, most patients had unilateral or bilateral CLBP, except one patient in each group presenting coccyx pain. 46% of patients (33 of 72) had previous spinal surgery before SI joint pain. The demographic data of patients between the groups were similar (Table 1). No patients encountered intraoperative or postoperative complications such as infections, hematoma, and neurologic impairment in the study. The navigation-assisted endoscopic rhizotomy operation time was significantly longer than cooled RFA (Table 2). The VAS of low back pain was significantly higher in the endoscopic group. (RFA group: 6.28 ± 1.28, Endoscope group: 7.25 ± 1.66; p < 0.05)
Table 1.
Comparison of patients on demographic and procedural characteristics
| Variable | RFA group (n = 36) | Endoscope group (n = 36) | p-value |
|---|---|---|---|
| Age | 63.69 ± 2.42 | 62.27 ± 2.37 | 0.905 |
| Sex | 0.198 | ||
| Male | 8 (22.2) | 13 (36.1) | |
| Female | 28 (77.8) | 23 (63.9) | |
| Height (cm) | 157.43 ± 1.32 | 158.54 ± 1.42 | 0.658 |
| Weight (kg) | 66.03 ± 1.80 | 63.92 ± 1.99 | 0.542 |
| BMI (kg/m2) | 26.7 ± 4.34 | 25.68 ± 4.19 | 0.838 |
| Smoking | 4 (11.1) | 1(2.7) | 0.177 |
| Alcohol | 2 (5.6) | 1(2.7) | 0.558 |
| Unilateral LBP | 15 (42) | 18 (49) | 0.77 |
| Bilateral LBP | 20 (56) | 17 (49) | |
| Coccyx pain | 1 (3) | 1 (2) | |
| Previous spine surgery | 14 (38.9) | 19 (54.2) | 0.240 |
| Operation time (min) | 39.08 ± 14.05 | 61.75 ± 23.55 | < 0.001* |
| Opioid use | |||
| Before | 8 (22.2) | 4 (11.1) | 0.209 |
| After | 6 (16.7) | 4 (11.1) | 0.499 |
Values are presented as number (%) or mean±standard deviation.
RFA, radiofrequency ablation; BMI, body mass index; LBP, low back pain.
p<0.05, statistically significant differences.
Table 2.
Visual analogue scale of back pain after treatment at different follow-up time
| Follow-up time | RFA group (n = 36) | Endoscope group (n = 36) | p-value |
|---|---|---|---|
| Preoperation | 6.28 ± 1.28 | 7.25 ± 1.66 | 0.008* |
| After 1 month | 2.03 ± 1.65 | 0.58 ± 1.13 | < 0.001* |
| After 3 months | 2.33 ± 1.53 | 0.75 ± 1.44 | < 0.001* |
| After 6 months | 3.28 ± 1.78 | 0.92 ± 1.68 | < 0.001* |
| After 12 months | 4.22 ± 2.40 | 1.14 ± 1.82 | < 0.001* |
| p-value | < 0.001 | < 0.001 |
Values are presented as mean±standard deviation.
RFA, radiofrequency ablation.
p<0.05, statistically significant differences.
After the operation, both groups showed statistically significant decreases in VAS of low back pain and ODI (p < 0.001). The improvement of SIJ pain and functional disability remained for the postoperative 1 year (Tables 3, 4). The full-endoscopic rhizotomy assisted with navigation was superior to cooled RFA regarding VAS reduction amplitude according to the between-group comparison at all follow-up times. The ODI scores at 1, 6, and 12 months were statistically significantly lower in the endoscopic group. During the 1-year follow-up, there was an upward trend of VAS and ODI in the cooled RFA group (Fig. 3). The Macnab criteria showed “excellent” in 86% and “good” in 11% of patients in the endoscopic group. On the contrary, the patients in the cooled RFA group reported “excellent” in 39% and “good” in 28% of patients (Fig. 4). Patients undergoing full-endoscopic rhizotomy assisted with navigation had a higher satisfaction rate after treatment (p < 0.05) (Table 4).
Table 3.
Functional outcomes after treatment
| ODI | RFA group (n = 36) | Endoscope group (n = 36) | p-value |
|---|---|---|---|
| Preoperation | 21.17 ± 3.90 | 20.8 ± 0 4.19 | 0.707 |
| After 1 month | 7.56 ± 6.03 | 4.64 ± 4.71 | 0.023* |
| After 3 months | 7.14 ± 5.98 | 4.89 ± 5.89 | 0.129 |
| After 6 months | 9.64 ± 7.92 | 5.11 ± 6.51 | 0.017* |
| After 12 months | 13.42 ± 8.79 | 5.25 ± 6.54 | < 0.001* |
| p-value | < 0.001 | < 0.001 |
Values are presented as mean±standard deviation.
ODI, Oswestry Disability Index; RFA, radiofrequency ablation.
p<0.05, statistically significant differences.
Table 4.
Satisfaction at 1-year follow-up
| 1-Year follow-up | RFA group (n = 36) | Endoscope group (n = 36) | p-value |
|---|---|---|---|
| Excellent | 14 | 31 | < 0.05 |
| Good | 10 | 4 | - |
| Fair | 6 | 1 | - |
| Poor | 6 | 0 | - |
RFA, radiofrequency ablation.
Fig. 3.
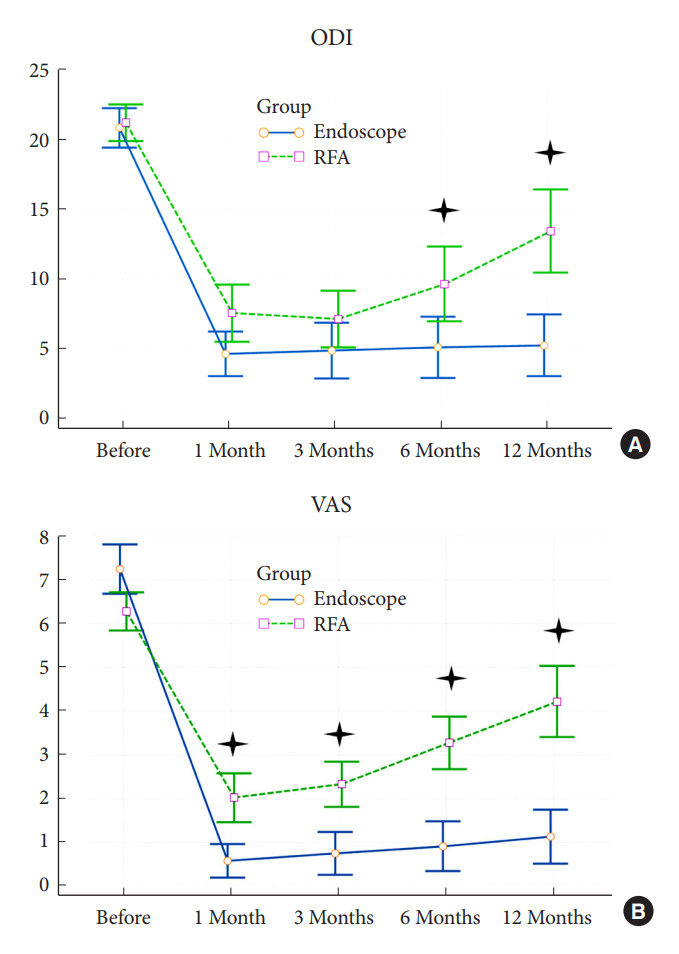
The clinical outcomes of the cooled radiofrequency ablation (RFA) group and the full-endoscopic rhizotomy group at each follow-up time. The values are given as median. (A) Oswestry Disability Index (ODI). (B) Visual analogue scale (VAS) scores.
Fig. 4.
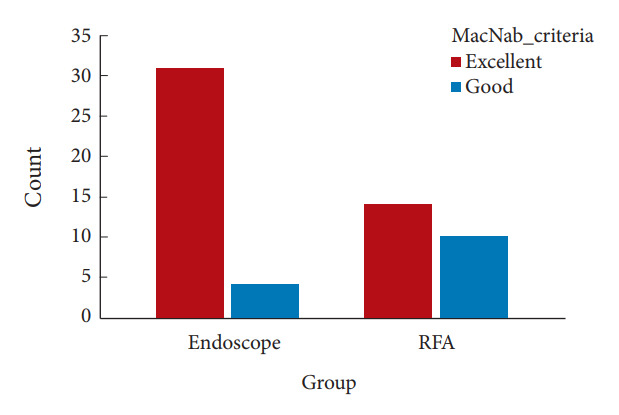
The satisfaction rate of each group at 1-year follow-up by MacNab criteria. Full-endoscopic rhizotomy group (Endoscope) showed 97% while the radiofrequency ablation (RFA) group showed 67% of the patient expressed “excellent” or “good.”
DISCUSSION
The current study was the first preliminary series of navigation-assisted full-endoscopic rhizotomy for SIJ pain. Although previous studies have shown the efficacy and safety of applying the endoscopic technique to treat SIJ pain, the imaging modality for endoscopic surgery is usually C-arm fluoroscopy. Recently, the computer-assisted navigation system has been widely applied in spinal surgeries. A technical report also demonstrated the feasibility of the navigation-assisted full-endoscopic rhizotomy. The CT-based navigation system provided clear 3D reconstruction images and guided the percutaneous procedures with tracked instruments. Nevertheless, there is no research to evaluate the reliability and durability compared with conventional RFA, a common intervention to treat SIJ pain patients. Therefore, the current series is essential to verify the roles of the new technologies.
The RFA has been an effective and standard treatment for SIJ pain. Ibrahim et al. [22] reported favorable outcomes regarding pain relief and functional improvement for up to 2 years. However, the classic RFA technique used fluoroscopic guidance rather than the CT-based navigation system. Besides, their technique included the ablation of L4–5 and L5–S1 medial branches, which were not innervating the SIJ complex. According to cadaveric research, the SIJ is mainly innervated by the lateral branch of the L5 dorsal rami, S1, S2, and S3 [11,17,23]. Therefore, the current series omitted the ablation or rhizotomy of medial branches of L4–5 and L5–S1 dorsal rami. Simplified procedures can be efficient without compromising results.
There have been various RFA techniques for treating SIJ pain (3 puncture method, strip lesion, and leapfrog technique) [24]. Besides, 3 types of RFA have been applied for different purposes or target nerves, including pulsed, thermal, and cooled RFA [24-27]. It is still being discussed which one has better efficacy over the others in treating SIJ pain. Shih et al. [26] claimed that there were no statistically significant differences between different types of RFA in their study. Although RFA significantly improves SI joint pain, the efficacy of RFA decreases with pain relapse. Some researchers hypothesized that the recurrence of pain resulted from nerve regeneration after lesioning [12,24,26,27]. Our results of the control group undergoing the RFA procedure also revealed similar trends. The pain and the functional disability relapsed gradually after 6 months to 12 months. Therefore, the authors emphasized the rhizotomy rather than ablation to resolve the SIJ pain. The lateral branches of the nerve roots might be smaller in diameter when they are away from the sacral foramen. Therefore, we recommend docking the endoscope as close as possible to the sacral foramen to identify the nerve branches. When nerve branches cannot be found surrounding the sacral foramen, ablation with a bipolar tip along the lateral border of the foramen is an alternative.
The endoscope development allows us to visualize sensory nerve fibers with a diameter between 0.21 to 1.51 mm [28]. The endoscopic procedure allows us to achieve maximum effect from the procedure, precisely targeting the L5 dorsal rami and lateral sacral branches from S1, S2, and S3 dorsal rami [13,22]. In our study, the VAS score was significantly higher in the endoscopic group. However, the difference in pain severity did not reduce the efficacy and durability of the endoscopic rhizotomy. Most of the patients who have received the endoscopic treatment did not experience the recurrence of pain over one year. Only 8% (3 out of 36) of patients in the endoscopic group experienced the recurrence as opposed to a 61% of recurrence rate after 1 year from RFA. Besides, the patient-reported satisfactory results also favored endoscopic treatment. More than 90% of the patients undergoing the endoscopic rhizotomy reported excellent or good satisfaction in the current study.
The CT-based navigation system can improve the accuracy and safety of image-guided procedures. The benefits are not only for patients but also for the surgical team. The innervation pattern of the L5 dorsal rami and the lateral sacral branches from S1, S2, and S3 dorsal rami are unique and various in each individual [13,28]. Our mission is to find those pain generators to perform the “cut-and-ablation” procedures at the paraforaminal area until the triggered pain decreases [17]. The conventional C-arm fluoroscopy is often insufficient to visualize the sacral foramen. 3D robotic C-arm navigation system lets us identify the foramen quickly. Anatomical landmarks such as L5, S1, S2, and S3 foramina were visualized on the constructed intraoperative virtual images of the SI joints. Surgeons can check the orientation and localization of the target immediately without interruption by adjusting the fluoroscopic device. Besides, radiation exposure due to C-arm fluoroscopy is a critical issue for the health of medical staff. Before and during the procedure, all surgical team members were free from radiation exposure during navigation-assisted procedures. The surgical team members can also work with better ergonomics without wearing a lead apron.
The navigation-assisted endoscopic procedure took longer operation time compared to the RFA procedure in the current study. Though the average operation time in the navigation-assisted endoscopic procedure was 61.8 minutes, the time can be decreased to about 30 minutes in the unilateral SIJ procedure when familiar with the registration and integration of the navigation system. Besides, to conduct a rhizotomy, it is necessary to visualize the nerve branches with the endoscope. The nerve branches are often hidden between the soft tissues and the ligaments [11]. Therefore, exploring the surgical field to find the nerve branches may cost time. Because patients were awake throughout the procedure, the surgeon can also use the bipolar coagulator to trigger the symptom to identify the pain generators. It is safer to monitor patients’ intraoperative response. If the patients complain of excessive or radiating pain, the surgeon can hold on to the procedure and re-evaluate the surgical orientation [17]. Estimated blood loss was minimal in the percutaneous procedures. The ambulatory surgery setting also avoided anesthetic risks. Therefore, no complications were noted, such as hematoma, motor nerve injury, or wound infections in the endoscopic procedures.
The current study supported that patients with failure after conservative treatment and relapsed symptoms after repeated SI joint injections are candidates for navigation-guided full-endoscopic rhizotomy. However, the navigation system might only be available in some hospitals. Fluoroscopy-assisted surgery can be an alternative imaging modality. From our experience, the ambulatory surgery setting enhances the application of the current technique. Patients with multiple comorbidities who cannot take general anesthesia for SI joint fusion or failure of previous RF ablation can benefit from the novel surgical treatment.
There were some limitations in the current study. First, this is a retrospective study with a small number of patients. Second, patients had diverse reasons for SI joint pain that other variables could confound. Third, most medical institutes do not offer a hybrid operation room or computer-assisted navigation system. Fourth, the imaging modalities were different between the group. The manipulated variables may affect perioperative parameters, such as surgical time. However, the bias resulting from the different imaging guidance was minor for the outcomes due to their assistant role. Finally, this is a pilot study for the current technique. Further prospective randomized studies or cohorts with long-term follow-ups are necessary to evaluate the applications of new techniques and technologies for SIJ pain.
CONCLUSION
The preliminary study indicates that navigation-assisted full-endoscopic rhizotomy is a feasible and effective alternative for SIJ pain treatment. Integrating intraoperative navigation with the endoscopic system ensures safe and accurate lesioning of target nerves without entering the foramen. The cohort of the current series proved the better durability of endoscopic rhizotomy compared with conventional RFA. Though long-term follow-up and randomized trials are necessary to confirm the superiority of the current endoscopic technique, it remains a potential to lead the trend of ambulatory surgery for SIJ pain treatment.
Acknowledgments
The authors thank the project assistant, Ms. Ying-Chieh Chen, for helping collect the materials and coordinate the program.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: CMC; Data curation: JHL, MYY, SWO, LWS; Formal analysis: JHL, SWJ, KTC; Methodology: MYY, K Chang, SWO; Project administration: CMC, LWS; Visualization: SWJ, KTC; Writing - original draft: CMC, JHL; Writing - review & editing: KTC.
REFERENCES
- 1.Cohen SP, Chen Y, Neufeld NJ. Sacroiliac joint pain: a comprehensive review of epidemiology, diagnosis and treatment. Expert Rev Neurother. 2013;13:99–116. doi: 10.1586/ern.12.148. [DOI] [PubMed] [Google Scholar]
- 2.Vleeming A, Schuenke MD, Masi AT, et al. The sacroiliac joint: an overview of its anatomy, function and potential clinical implications. J Anat. 2012;221:537–67. doi: 10.1111/j.1469-7580.2012.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiapour A, Joukar A, Elgafy H, et al. Biomechanics of the sacroiliac joint: anatomy, function, biomechanics, sexual dimorphism, and causes of pain. Int J Spine Surg. 2020;14(Suppl 1):3–13. doi: 10.14444/6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101:1440–53. doi: 10.1213/01.ANE.0000180831.60169.EA. [DOI] [PubMed] [Google Scholar]
- 5.Foley BS, Buschbacher RM. Sacroiliac joint pain: anatomy, biomechanics, diagnosis, and treatment. Am J Phys Med Rehabil. 2006;85:997–1006. doi: 10.1097/01.phm.0000247633.68694.c1. [DOI] [PubMed] [Google Scholar]
- 6.Gartenberg A, Nessim A, Cho W. Sacroiliac joint dysfunction: pathophysiology, diagnosis, and treatment. Eur Spine J. 2021;30:2936–43. doi: 10.1007/s00586-021-06927-9. [DOI] [PubMed] [Google Scholar]
- 7.Szadek KM, van der Wurff P, van Tulder MW, et al. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10:354–68. doi: 10.1016/j.jpain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Laslett M, Young SB, Aprill CN, et al. Diagnosing painful sacroiliac joints: A validity study of a McKenzie evaluation and sacroiliac provocation tests. Aust J Physiother. 2003;49:89–97. doi: 10.1016/s0004-9514(14)60125-2. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante FM, King LF, Roche EA, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26:137–42. doi: 10.1053/rapm.2001.21739. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan G, Hehar P, Loomba V, et al. A Randomized controlled trial of fluoroscopically-guided sacroiliac joint injections: a comparison of the posteroanterior and classical oblique techniques. Neurospine. 2019;16:317–24. doi: 10.14245/ns.1836122.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreyfuss P, Henning T, Malladi N, et al. The ability of multisite, multi-depth sacral lateral branch blocks to anesthetize the sacroiliac joint complex. Pain Med. 2009;10:679–88. doi: 10.1111/j.1526-4637.2009.00631.x. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SP, Hurley RW, Buckenmaier CC, 3rd, et al. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109:279–88. doi: 10.1097/ALN.0b013e31817f4c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi WS, Kim JS, Ryu KS, et al. Endoscopic radiofrequency ablation of the sacroiliac joint complex in the treatment of chronic low back pain: a preliminary study of feasibility and efficacy of a novel technique. Biomed Res Int. 2016;2016:2834259. doi: 10.1155/2016/2834259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi G, Lee SH, Bhanot A, et al. Modified transcorporeal anterior cervical microforaminotomy for cervical radiculopathy: a technical note and early results. Eur Spine J. 2007;16:1387–93. doi: 10.1007/s00586-006-0286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TT, Johnson JP, Pashman R, et al. Minimally invasive spinal surgery with intraoperative image-guided navigation. Biomed Res Int. 2016;2016:5716235. doi: 10.1155/2016/5716235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SJ, Hsiao MC, Lee JH, et al. How I do it? Full endoscopic lumbar rhizotomy for chronic facet joint pain due to failed back surgery syndrome. Acta Neurochir (Wien) 2022;164:1233–7. doi: 10.1007/s00701-021-05042-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Chen KT, Chang KS, et al. How I do it? Fully endoscopic rhizotomy assisted with three-dimensional robotic C-arm navigation for sacroiliac joint pain. Acta Neurochir (Wien) 2021;163:3297–301. doi: 10.1007/s00701-020-04682-2. [DOI] [PubMed] [Google Scholar]
- 18.Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976) 1996;21:2594–602. doi: 10.1097/00007632-199611150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16:142–52. doi: 10.1179/jmt.2008.16.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maus T. Imaging the back pain patient. Phys Med Rehabil Clin N Am. 2010;21:725–66. doi: 10.1016/j.pmr.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Loh E, Burnham TR, Burnham RS. Sacroiliac joint diagnostic block and radiofrequency ablation techniques. Phys Med Rehabil Clin N Am. 2021;32:725–44. doi: 10.1016/j.pmr.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim R, Telfeian AE, Gohlke K, et al. Endoscopic radiofrequency treatment of the sacroiliac joint complex for low back pain: a prospective study with a 2-year follow-up. Pain Physician. 2019;22:E111–8. [PubMed] [Google Scholar]
- 23.Cox RC, Fortin JD. The anatomy of the lateral branches of the sacral dorsal rami: implications for radiofrequency ablation. Pain Physician. 2014;17:459–64. [PubMed] [Google Scholar]
- 24.Aydin SM, Gharibo CG, Mehnert M, et al. The role of radiofrequency ablation for sacroiliac joint pain: a meta-analysis. PM R. 2010;2:842–51. doi: 10.1016/j.pmrj.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Young AC, Deng H, Opalacz A, et al. A retrospective analysis of sacroiliac joint pain interventions: intraarticular steroid injection and lateral branch radiofrequency neurotomy. Pain Physician. 2022;25:E341–7. [PubMed] [Google Scholar]
- 26.Shih CL, Shen PC, Lu CC, et al. A comparison of efficacy among different radiofrequency ablation techniques for the treatment of lumbar facet joint and sacroiliac joint pain: A systematic review and meta-analysis. Clin Neurol Neurosurg. 2020;195:105854. doi: 10.1016/j.clineuro.2020.105854. [DOI] [PubMed] [Google Scholar]
- 27.Bayerl SH, Finger T, Heiden P, et al. Radiofrequency denervation for treatment of sacroiliac joint pain-comparison of two different ablation techniques. Neurosurg Rev. 2020;43:101–7. doi: 10.1007/s10143-018-1016-3. [DOI] [PubMed] [Google Scholar]
- 28.Roberts SL, Burnham RS, Ravichandiran K, et al. Cadaveric study of sacroiliac joint innervation: implications for diagnostic blocks and radiofrequency ablation. Reg Anesth Pain Med. 2014;39:456–64. doi: 10.1097/AAP.0000000000000156. [DOI] [PubMed] [Google Scholar]



