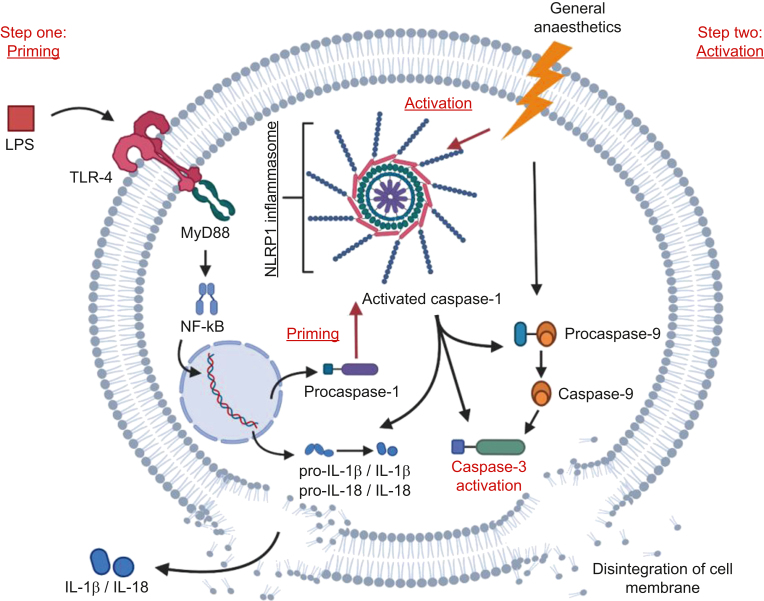
Fig 6.
Proposed two-hit hypothesis of worsened anaesthetic neurotoxicity when combined with inflammation. The first hit is provided when LPS crosses the blood–brain barrier and binds to TLR-4 receptors, activating the MyD88–Nf-kB axis. This leads to upregulation of genes that code for the pro-forms of IL-1β and IL-18, as well as pro-caspase-1 and other inflammasome components such as NLRP1, and also inducing the assembly of caspase-1/NLRP1 inflammasome complex. With inflammasome assembled, pro-caspase-1 undergoes auto-proteolytic activation to caspase-1. The second hit is provided by the general anaesthetic, which activates the previously primed inflammasome, in addition to triggering the apoptotic cascade via caspase-9/caspase-3 cleavage. Primed and activated inflammasome and caspase-1 lead to further cleavage of caspase-9 and caspase-3, therefore exacerbating the apoptotic death of neurones. Secretion of IL-1β and IL-18 processed by caspase-1 could promote neuronal apoptosis, thus facilitating neuronal death. IL, interleukin; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response 88; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP1, NLR family pyrin domain containing 1; TLR, Toll-like receptor.