TABLE 2.
Some labeling synthons prepared from aryliodonium ylides
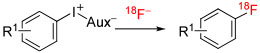
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Labeling Synthon | Precursor |
Labeling Conditions |
Yield,b % | References | ||||
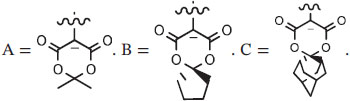
| R1 | Aux−a | Mode | Solvent | °C | min | ||||
| 1 | [18F]3-Fluorobromobenzene | 3-Br | C | Batch | DMF | 130 | 10 | 72 ± 3 | Yuan et al115 |
| 2 | [18F]4-Fluoroiodobenzene | 4-I | A | Batch | DMSO | 110 | 15 | 70 ± 10 | Kügler et al101 |
| 3 | [18F]3-Fluorobenzaldehyde | 3-CHO | B | Batch | DMF | 120 | 10 | 7-52 | Petersen et al116 |
| 4 | [18F]3-Fluorobenzyl azide | 3-CH2N3 | B | Batch | DMF | 120 | ns | 69 ± 8 | Wang et al117 |
| 5 | [18F]4-Fluorobenzyl azide | 4-CH2N3 | B | Batch | DMF | 120 | 10 | 70 ± 4 (52 ± 2) | |
| 6 | [18F]4-Fluorobenzyl azide | 4-CH2N3 | B | Batch | DMF | 120 | 10 | (25 ± 10) c | Rotstein et al67 |
| 7 | [18F]4-Fluorobenzyl azide | 4-CH2N3 | B | MR | DMF | 210 | ~1 | 68 ± 5 (24 ± 0) | Calderwood et al75 |
| 8 | [18F]3-Fluorobenzoic acid methyl ester | 3-CO2Me | B | Batch | DMF | 120 | 10 | 77 ± 7 | Rotstein et al67 |
| 9 | [18F]2-(3-Fluorophenyl)ethylamined | 3-(CH2)2NHBoc | A | Batch | DMF | 110 | 10 | ~70 | Drerup et al118 |
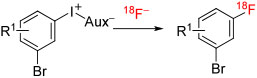
| |||||||||
| 10 | [18F]1-(2-Azidoethoxy)-4-bromo-2-fluorobenzene | 2-(O(CH2)2N3) | B | Batch | DMF | 120 | 10 | 69 ± 2 | Wang et al117 |
| 11 | [18F]1-((2-Azidoethoxy)methyl)-4-bromo-2-fluorobenzene | 2-(CH2 O(CH2)2N3) | B | Batch | DMF | 120 | 10 | 90 ± 2 | Wang et al117 |
Abbreviations: DMF, dimethylformamide; DMSO, dimethyl sulfoxide; MR, microreactor (microfluidic); ns, not specified.
Values in plain type are yields before isolation. Values in bold and parentheses are yields after isolation.
Uncorrected for decay.
Process is 2 steps. Data are for the radiofluorination step.
