TABLE 5.
Radiotracers prepared from aryliodonium ylides
| Entry | Radiotracer Target |
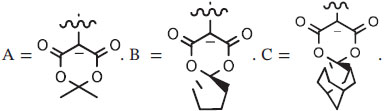
Precursor (ArI+Aux−) |
Labeling conditions |
Yield,b % | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Structure | ArI+ | Aux−a | Mode | Solvent | °C | min | |||
| 1 | [18F]4-FPPMP |
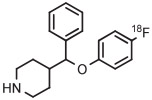
|
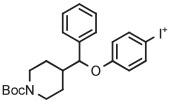
|
A | Batch (2 steps) | MeCN | 130 | 20 | 20 | Cardinale et al94 |
| 2 | [18F]3-FPPMP |
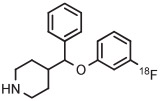
|
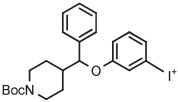
|
A | Batch (2 steps) | MeCN | 120 | 20 | (20 ± 5) | Cardinale et al94 |
| 3 | [18F]FPEB |
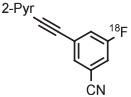
|
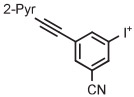
|
B | Batch (1 step) | DMF | 80 | 5 | 49 ± 6 | Stephenson et al139 |
| 4 | [18F]FPEB |
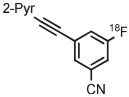
|
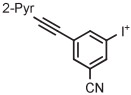
|
B | MR (1 step) | DMF | 200 | ~1 | (20 ± 5) | Stephenson et al139 |
| 5 | [18F]LY2459989 |
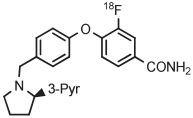
|
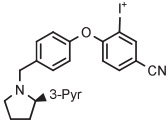
|
B | MR (2 steps) | DMF | 120 | 5 | 76 | Cai et al140 |
| 6 | [18F]LY2459989 |
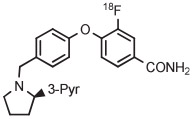
|
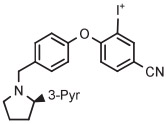
|
B | Batch (2 steps) | DMF | 80 | 5 | 49 (30-43) | Cai et al140 |
| 7 | [18F]PFH |
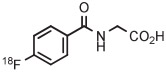
|
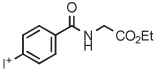
|
B | Batch (2 steps) | DMF | 130 | 10 | (46 ± 3) | Nkepang et al142 |
| 8 | [18F]4-FPhe |
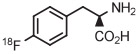
|
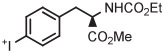
|
B | Batch (2 steps) | DMF | 120 | 10 | 55 ± 4 | Rotstein et al67 |
| 9 | [18F]CIMBI-198 |
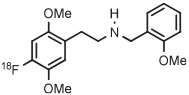
|
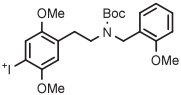
|
B | Batch with TEMPO (2 step) | DMF | 135 | 20 | 12 ± 3 (2) | Petersen et al143 |
| 10 | 18F-Labeled 5-HT2A receptor radiotracer |
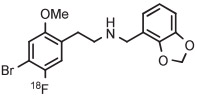
|
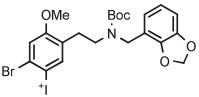
|
B | Batch (2 steps) with TEMPO | DMF | 135 | 20 | 76-77 (10-12) | Petersen et al143 |
| 11 | [18F]Lorlatinib analog |
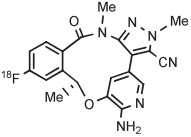
|
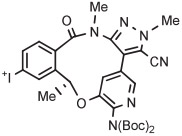
|
B | Batch (2 steps) | DMF | 80 | 10 | (14) c | Collier et al144 |
| 12 | [18F]FDPA |
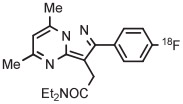
|
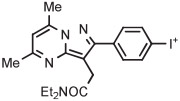
|
C | Batch (1 step) | DMF | 120 | 15 | 40 ± 3 | Wang et al145 |
| 13 | [18F]FDPA |
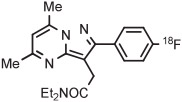
|
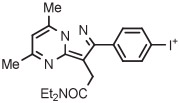
|
C | Batch (1 step) | MeCN | 120 | 12 | 45 ± 8 | Wang et al145 |
| 14 | [18F]Safinamide |
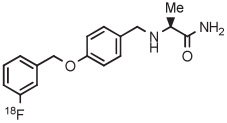
|
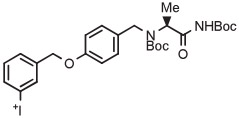
|
C | Batch (2 steps) | DMF | 120 | 10 | 15 | Rotstein et al93 |
| 15 | [18F]FMT |
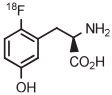
|
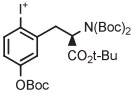
|
C | Batch (2 steps) | DMF | 120 | 20 | 60 (12)cb | Rotstein et al93 |
| 16 | [18F]MFBG |
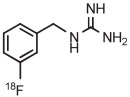
|
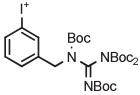
|
C | Batch (2 steps) | DMF | 120 | 10 | 70 (14)c | Rotstein et al93 |
Abbreviations: DMF, dimethylformamide; [18F]FMT, [18F]l-2-fluoro-meta-tyrosine; MeCN, acetonitrile; [18F]MFBG, [18F]1-(3-Fluorobenzyl)guanidine; TEMPO, 2,2,6,6-tetramethyl-1-piperidinyloxyl.
Values in bold and parentheses are yields after isolation.
Not decay corrected.
