Abstract
Past studies in autism spectrum disorder (ASD) indicate atypical peripheral physiological arousal. However, the conditions under which these atypicalities arise and their link with behavioral emotional expressions and core ASD symptoms remain uncertain. Given the importance of physiological arousal in affective, learning, and cognitive processes, the current study examined changes in skin conductance level ΔSCL) in 41 toddlers with ASD (mean age: 22.7 months, SD: 2.9) and 32 age-matched toddlers with typical development (TD) (mean age: 21.6 months, SD: 3.6) in response to probes designed to induce anger, joy, and fear emotions. The magnitude of ΔSCL was comparable during anger (P = 0.206, d = 0.30) and joy (P = 0.996, d = 0.01) conditions, but significantly lower during the fear condition (P = 0.001, d = 0.83) in toddlers with ASD compared to TD peers. In the combined samples, ΔSCL positively correlated with intensity of behavioral emotional expressivity during the anger (r[71] = 0.36, P = 0.002) and fear (r[68] = 0.32, P = 0.007) conditions, but not in the joy (r[69] = −0.15, P = 0.226) condition. Finally, ΔSCL did not associate with autism symptom severity in any emotion-eliciting condition in the ASD group. Toddlers with ASD displayed attenuated ΔSCL to situations aimed at eliciting fear, which may forecast the emergence of highly prevalent internalizing and externalizing problems in this population. The study putatively identifies ΔSCL as a dimension not associated with severity of autism but with behavioral responses in negatively emotionally challenging events and provides support for the feasibility, validity, and incipient utility of examining ΔSCL in response to emotional challenges in very young children.
Keywords: autism, electrodermal activity, emotional arousal, fear, toddlers
Lay Summary:
Physiological arousal was measured in toddlers with autism exposed to frustrating, pleasant, and threatening tasks. Compared to typically developing peers, toddlers with autism showed comparable arousal responses to frustrating and pleasant events, but lower responses to threatening events. Importantly, physiological arousal and behavioral expressions were aligned during frustrating and threatening events, inviting exploration of physiological arousal to measure responses to emotional challenges. Furthermore, this study advances the understanding of precursors to emotional and behavioral problems common in older children with autism.
Introduction
Although autism spectrum disorder (ASD) is characterized diagnostically by difficulties in social interactions and the presence of restricted behaviors and activities [American Psychiatric Association, 2013], emotional difficulties constitute a frequently co-occurring feature of ASD, both in young children as well as in adolescents and adults [Garon et al., 2016;Green, Ben-Sasson, Soto, & Carter, 2012]. Parents of toddlers with ASD report a predisposition to negative emotions and less intense displays of positive emotions in their young children compared to parents of toddlers with typical development (TD) and developmental delays (DDs) [De Pauw, Mervielde, Van Leeuwen, & De Clercq, 2011;Garon et al., 2009;Macari, Koller, Campbell, & Chawarska, 2017]. These emotional vulnerabilities show considerable continuity from the second to fourth years of life [Macari et al., 2017], and may forecast emergence of internalizing and externalizing mental health problems [Gadow, DeVincent, & Schneider, 2008;Leyfer et al., 2006;Salazar et al., 2015; Simonoff et al., 2008;Wood & Gadow, 2010]. More granular and direct behavioral measurement of intensity of emotional expression in toddlers with ASD suggests that in response to naturalistic challenges (e.g., restraint in a car seat), affected toddlers respond with more intense anger and frustration than toddlers with other disabilities but not typical controls [Macari et al., 2018]. They also show attenuated intensity of fear response to potentially threatening objects (e.g., mechanical tarantula) [Macari et al., 2018]. However, neither intensity of emotional expression of anger nor fear appear to be associated with autism severity [Macari et al., 2017;Macari et al., 2018], suggesting independence between emotional difficulties and autism phenotypes.
While parent report and direct measures of emotional expression are informative, there is a possibility at the behavioral level that children with ASD exhibit emotions through vocal and facial channels in an atypical or inconsistent manner [Costa, Steffgen, & Samson, 2017; Shalom et al., 2006]. In this context, it is not clear whether attenuated fear responses observed in naturalistic situations relate to a limited capacity to express rather than experience fear. To address this issue, several methodological and conceptual models of atypical emotional experience in ASD have increasingly been proposed in an attempt to unite and associate the numerous findings of atypical motivation, neural circuitry, information processing, as well as physiological responses in ASD [Mazefsky & White, 2014]. Considering the tight links between peripheral nervous system reactivity and intensity of emotional expression in younger and in nonverbal children [Ekman, 1992; Fox, 1989; Lazarus, 1991; Stifter, Fox, & Porges, 1989], experiential facets of intensity of emotional expression can be accessed in part through measures of the autonomic nervous system, including electrodermal activity (EDA). Brain areas demonstrated to associate with EDA when evaluating emotionally significant events and stimuli include the amygdala, orbitofrontal cortex, ventromedial prefrontal cortex, right inferior parietal region, and anterior cingulate [Dawson, Schell, & Filion, 2000]. Thus, EDA is believed to index sympathetic “fight-or-flight” nervous system activity to prepare the body for action in response to excitement and stress [Langley, 1921]. While basal skin conductance level (SCL) measures of EDA are informative regarding general states of arousal over time, within-person event-related changes (i.e., EDA reactivity) in SCL (i.e., ΔSCL) provide more reliable physiological responses to discrete internal or external events over short periods of exposure [Ekman, 1992; Kreibig, 2010]. Accordingly, ΔSCL provides a versatile measure of peripheral sympathetic nervous system reactivity and has been reliably measured in very young infants including typically developing newborns and children with ASD [Goodwin, 2016; Prince et al., 2017; Storm, 2000] who may be less tolerant of cardiovascular measurements requiring electrodes placed on the chest.
Numerous induction techniques using socio-emotional stimuli, sensory presses, and stress-inducing triggers have been employed in a growing number of studies involving children above 3 years of age and adolescents with ASD to study physiological arousal using EDA [Lydon et al., 2016]. However, many laboratory-based studies rely on computer monitor-administered probes and yield mixed results. Physiological studies examining children with ASD in comparison to TD peers demonstrate significantly weaker physiological arousal to threatening social and nonsocial pictures (e.g., crying face, angry face, shark) [Blair, 1999], atypical responses to a non-reward phase of a reward task [Neuhaus, Bernier, & Beauchaine, 2015], lower arousal to a social aggression-based computer game [Schneider et al., 2015], and greater arousal to eye contact [Kylliäinen & Hietanen, 2006]. However, no differences between children with and without ASD in physiological arousal were found in other studies employing faces with direct gaze [Kaartinen et al., 2012; Louwerse et al., 2013], face stimuli [Cohen, Masyn, Mastergeorge, & Hessl, 2015;Shalom et al., 2006], distress cues (e.g., crying face) [Blair, 1999], or the stress-inducing Stroop task [Kushki et al., 2013].
Moreover, conflicting findings are reported in studies that employ more ecologically valid induction probes. For instance, when exposed to their mother, TD children show enhanced EDA responses during live exchanges of gaze compared to facing an object such as a paper cup, while children with ASD show a lack of difference between EDA responses to these stimuli of familiar human face and object [Hirstein, Iversen, & Ramachandran, 2001]. However, another study reported no differences in EDA (sympathetic index) and vagal tone (parasympathetic index) in response to the Trier social stress task [Kirschbaum, Pirke, & Hellhammer, 1993] in children with and without ASD [Levine et al., 2012]. It is important to note that despite similar sympathetic and parasympathetic responses observed in both ASD and TD groups, the ASD group showed a decrease in cortisol level following the Trier stressor task compared to the TD group who showed an increase in cortisol level [Levine et al., 2012].
A small number of studies have examined EDA in toddlers with ASD. In response to play-based probes administered in the context of a social interaction aimed at eliciting communicative bids (“communicative temptations”), toddlers with ASD exhibited more pronounced EDA reactivity compared to TD controls [Prince et al., 2017]. Conversely, toddlers with ASD displayed comparable EDA reactivity as TD peers in response to sensory stimuli in visual, auditory, tactile, and olfactory modalities as well as visual displays of repetitive movement [McCormick et al., 2014].
Although the majority of studies employing indices of peripheral physiological arousal indicate some abnormalities to various stimuli, the direction in which this atypicality lies (i.e., hyporeactivity or hyperreactivity) remains uncertain [White et al., 2014]. The discrepancies found among studies may be due to several factors, including differences in induction type, measurement, and analysis methods; age and developmental level [Lydon et al., 2016]; and general arousal level before stimulation [Cohen et al., 2015;Palkovitz & Wiesenfeld, 1980]. Similarly, the nature (intensity and valence) of emotional elicitation may also explain some of the inconsistencies observed in the literature. The use of different provocations to elicit various emotions such as anger, fear, and happiness has often been done in isolation (e.g., Taylor aggression paradigm, [Schneider et al., 2015]; Stranger procedure [Naber et al., 2007]). Moreover, although large EDA responses can be obtained when participants are exposed to pleasant stimuli [Bradley & Lang, 2000], examining sympathetic nervous system responses to joy-eliciting stimuli early in autism has not been done extensively [McCormick et al., 2014;Prince et al., 2017]. Finally, no published studies have yet compared behavioral and physiological data collected simultaneously in toddlers with ASD during emotion-inducing conditions. Thus, it is not clear if peripheral physiological responses of young children with ASD to emotion-inducing challenges are atypical or more heterogeneous, and whether they are congruent with intensity of observable behavioral responses and measures of autism severity.
The present study simultaneously examines peripheral physiological and behavioral reactivity in toddlers newly diagnosed with ASD and TD controls in response to standardized probes aimed to elicit anger, joy, and fear. The laboratory temperament assessment battery (Lab-TAB)—Locomotor Version [Gagne, Van Hulle, Aksan, Essex, & Goldsmith, 2011;Goldsmith & Rothbart, 1999] probes are standardized and similar to situations toddlers face regularly in real life including anger-evoking events related to goal-blocking, playful events such as puppet shows, and fear-inducing events involving unfamiliar objects and situations. The Lab-TAB probes have been previously used to examine emotional reactivity to anger- and fear-eliciting situations in children with ASD [Costa et al., 2017;Jahromi, Meek, & Ober-Reynolds, 2012; Scherr, Hogan, Hatton, & Roberts, 2017], but evidence regarding joy reactivity is still limited [Macari et al., 2018].
The present study examined whether: (1) the magnitude of EDA responses to anger, joy, and fear-inducing conditions differs between toddlers with ASD and TD controls; (2) intensity of EDA responses associates with intensity of behavioral responses expressed through vocal and facial channels, and (3) intensity of EDA responses correlates with severity of autism symptoms in social affective and restricted and repetitive behavior domains. Based on prior work indicating that toddlers with ASD show more intense anger behavioral responses compared to DD toddlers (but not TD controls) [Macari et al., 2018], and exhibit less intense expression of fear compared to both DD and TD groups, we hypothesized that toddlers with ASD would display a similarly contrasting pattern of EDA responses to frustrating situations (Anger condition) vs. threatening (Fear condition) behavior. More precisely, we predicted greater EDA responses to anger-inducing probes, lower EDA reactivity to fear-inducing probes, and remained exploratory regarding changes in response to joy-inducing probes. Secondly, considering mixed reports regarding links between behavioral and physiological responses [Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005], we explored the correlation between EDA reactivity and the intensity of emotional expressivity (iEE) in the anger-, joy-, and fear-inducing conditions in toddlers across the spectrum of social ability-disability. Finally, although previous work in children of 4–11 years of age with moderate to high ASD symptom levels [Fenning et al., 2017] has shown links between greater physiological arousal variability in response to extended naturalistic social situations and severity of ASD symptoms, more recent work [Macari et al., 2018] suggests that intensity of behavioral responses to nonsocial triggers aimed to elicit negative emotions in toddlers with ASD does not relate to autism symptom severity. Thus, our final analysis was to explore whether the magnitude of physiological responses to emotion-inducing probes vary with the severity of autism symptoms.
Methods
Participants
The study was approved by the Human Investigation Committee of the Yale School of Medicine, and informed written consent was obtained from all parents prior to testing the children. Participants included 41 toddlers with ASD (32 males) and 32 TD toddlers (21 males). The participants with ASD were consecutive referrals to the Yale Toddler Developmental Disabilities Clinic. TD participants were recruited through online advertisements and community outreach. Developmental skills were assessed using the Mullen Scales of Early Learning (MSEL; [Mullen, 1995] and severity of autism symptoms was assessed using the Autism Diagnostic Observation Schedule-2 Toddler Module (ADOS-T) [Lord et al., 2012] administered by a PhD-level psychologist research-reliable on the measure. Adaptive skills were assessed using parent interviews for the Vineland Adaptive Behavior Scales—Second Edition (VABS-2) [Sparrow, Balla, Cicchetti, Harrison, & Doll, 1984]. To be included in the ASD sample, the child had to receive a clinical best estimate diagnosis of ASD by a team of expert clinicians based on DSM-5 criteria, and on all available results of assessments listed above, as well as developmental and medical history. Toddlers were classified as TD if they had verbal and nonverbal developmental quotient (DQ) scores on the MSEL above 80 and a total ADOS-T calibrated severity score of 3 or lower, with an expert clinician confirming the assignation. Children with a history of prematurity or known genetic abnormalities were not included in the study. In 62% of toddlers with ASD, diagnosis was based on follow-up evaluation when the child was over 30 months old (mean = 38.12 months, SD = 2.36). The remaining cases received their diagnosis around 2 years of age (mean = 21.46 months, SD = 3.27). Prospective studies report over 90% stability of ASD diagnosis from the second year into preschool age [Chawarska, Klin, Paul, Macari, & Volkmar, 2009; Guthrie, Swineford, Nottke, & Wetherby, 2013;Kim, Macari, Koller, & Chawarska, 2015], thus, if any diagnostic shifts occurred in the ASD group, they were likely to affect only a small number of cases (i.e., not likely to alter the present results). The two groups did not differ in their distribution of gender (P = 0.257) or age (P = 0.171). The groups differed in a predictable manner regarding severity of autism symptoms as well as MSEL verbal and nonverbal DQ (see full descriptive statistics in Table 1). Finally, it should be noted that the behavioral responses (but not the peripheral physiological responses) of 45% of the participants included in this study have been published by Macari et al. [2018].
Table 1.
Participant Characteristics
| ASD (n = 41) |
TD (n = 32) |
Contrast | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Sex ratio (%male) | 78 | 66 | ASD = TD (P = 0.257) |
| Chronological age in months | 22.68 (2.94) | 21.58 (3.64) | ASD = TD (p = 0.171) |
| MSEL—Verbal DQ | 52.57 (25.04) | 112.16 (18.42) | ASD < TD (P < 0.001) |
| MSEL—Nonverbal DQ | 82.69 (13.41) | 107.24 (14.33) | ASD < TD (P < 0.001) |
| ADOS-2 Toddler—SA | 13.34 (4.61) | 1.97 (1.79) | ASD > TD (P < 0.001) |
| ADOS-2 Toddler—RRB | 3.80 (1.63) | 0.66 (0.75) | ASD > TD (P < 0.001) |
| ADOS-2 Toddler—total score | 17.15 (5.48) | 2.63 (1.83) | ASD > TD (P < 0.001) |
Abbreviations: ADOS-2, Autism Diagnostic Observation Schedule, Second Edition (Total score of Toddler module); AE, age equivalent; ASD, autism spectrum disorder; DQ, developmental quotient; MSEL, Mullen Scales of Early Learning Composite Standard Score; RRB, restricted and repetitive behaviors; SA, social affect; TD, typical development.
Stimuli and Procedure
Laboratory temperament assessment battery.
To assess in vivo emotional response intensity across facial and vocal modalities elicited by positively (joy) and negatively (anger and fear) valenced probes, we employed a modified set derived from the Lab-TAB [Goldsmith & Rothbart, 1999]. Each child was administered a set of nine standardized probes aimed to elicit anger, joy, and fear (three probes per emotional condition, presented in the following order with the Joy probes occurring between the Anger and Fear probes to avoid exposing toddlers to too many successive negative emotion-eliciting probes). The Anger probes comprised Car Seat (child briefly restrained in a car seat, 1 × 30 sec); End of Line (desirable toy out of reach, 2 × 30 sec); and Restraint (desirable toy out of reach with child’s arms gently restrained by parent, 2 × 30 sec). The Joy probes comprised Bubbles (large displays of bubbles, 3 × 10 sec), Puppets (two hand puppets shown in a peek-a-boo manner 3 × 10 sec), and Penguins (demonstration of a “penguin race” rollercoaster toy, 3 × 10 sec). Finally, the Fear probes comprised Spider (mechanical tarantula-like spider crawling toward the child, 3 × 10 sec), Masks (experimenter wearing three different Halloween masks with a black full-length cape opening the door and standing inside the room 2.5 m away facing the child, 3 × 10 sec), and Dinosaur (mechanical dinosaur with red light-up eyes approaching the child, 3 × 10 sec). While the Fear probes involved encounters with potentially threatening situations, the Joy and Anger probes aimed to induce emotions from two ends of the positive—negative continuum. In both situations, an interesting object or activity was either presented (access to desired object; Joy condition) or removed (access to object denied; Anger condition). To diminish carry-over effects from one probe to another, standardized inter-stimulus breaks were introduced between probes and a new probe began only when the child appeared to return to a behaviorally neutral state. Such short baselines and periods of inter-stimulus breaks have been used in previous studies involving participants from the general population [Benedek & Kaernbach, 2010; Haney & Euse, 1976; Kreibig, 2010]. The experiments were video recorded and coded off-line for peak iEE (anger, joy, fear), thus capturing the tendency to respond to stimuli using extreme behavioral responses [Planalp, Van Hulle, Gagne, & Goldsmith, 2017].
Electrodermal reactivity.
Peripheral physiological arousal reactivity was measured in EDA micro-siemens recorded from the left ankle during the Lab-TAB tasks using an Affectiva Q-Sensor—a small ankle-mounted band that captures EDA, skin surface temperature, and 3D motion at 32 Hz—recognized to provide a reliable measure of peripheral sympathetic nervous system arousal level in children with autism [Goodwin, 2016; Prince et al., 2017]. The Q sensor was augmented with snap-in gelled adhesive electrodes to reduce motion artifact and boost signal amplitude. To promote quality EDA recordings, the following data acquisition considerations were implemented: (1) use of a moderately illuminated room with minimal decoration; (2) provision of a non-caffeinated liquid refreshment before the experiment to control for hydration status; (3) a consistent ambient room temperature; and (4) a 15-min warm-up period to allow the sensor to adapt to the toddlers’ body temperature prior to study data collection. Onset and offset of Lab-TAB probes were time-stamped and logged according to National Institute of Standards and Technology criteria, enabling careful synchronization of Lab-TAB video recordings and corresponding segmented SCL data streams. Intensity of both physiological and behavioral responses to probes was simultaneously recorded.
Intensity of emotional expressivity.
Intensity of anger, joy, and fear expressions in separate facial and vocal channels were video coded offline for valence (positive vs. negative) and peak intensity (none, mild, moderate, high) for each probe during Anger, Joy, and Fear eliciting conditions. All participants with physiological responses also contributed behavioral data. Coders blind to the child’s diagnostic status used a coding system for facial expressions based on the AFFEX system [Izard, Huebner, Risser, & Dougherty, 1980], consisting of graded descriptions of facial movements specifying the level of intensity of facial expressions during each trial. Intensity of positive and negative vocalizations was rated using codes based on the Lab-TAB [Goldsmith & Rothbart, 1999]. Please see Macari et al. [2018] for complete descriptions of the codes. All coders were blind to the physiological patterns of responses and had established reliability with a master coder (intraclass correlation coefficient [ICC] ≥ 0.75) before starting to code. Fourteen percentage of the data files were randomly selected and double coded for the three emotion eliciting conditions to maintain reliability (0.77 < ICCs <0.94). Composite raw scores for overall intensity of expressivity of anger, joy, and fear responses were averaged across facial and vocal channels for each trial of the probes, and further averaged into a mean iEE for each emotion-eliciting condition.
Data Reduction
First, behavior camera recordings and rigorous quality control procedures prior to analysis (i.e., completion of each of the nine probes of the Lab-TAB, parental interfer-ence (e.g., pointing, referring to object), and technical issues (e.g., malfunction of the mechanical spider or dinosaur) were coded to ensure participants produced useable data. Using the methods by [Kleckner et al., 2017], EDA data were then automatically pre-processed (e.g., low pass filtering to remove noise with cutoff frequencies at 0 and 0.35 Hz and designed for a sampling frequency of 32 Hz) by combining an inhouse data pipeline programmed in MATLAB (MathWorks) and R [Team R Core, 2013], followed by signal quality assessments (e.g., floor or ceiling artifact removal, below 0.05 μS) [Boucsein, 2012] and above 60 μS (Affectiva Q Sensor maximum value), skin temperature check to assess loss of contact with the sensor (30°C< SCL < 40°C), and response rate check to remove the presence of physiologically unrealistic EDA signal such as jumps and movement artifacts, Four additional toddlers (ASD: n = 3, TD: n = 1) provided invalid EDA data according to these procedures. The remaining participants provided a high proportion of probes with valid EDA data (valid probes: ASD: 93.65%, TD: 96.18%). The final sample size ratio per group (ASD/TD) in each condition was as follows: 41/32 in the Anger condition, 40/31 in the Joy condition, and 39/31 in the Fear condition.
Basal SCL without stimulation was computed by averaging across the 10-sec baseline periods captured at the start of each probe. To assess changes in SCL evoked during emotional conditions experienced by participants, we quantified peripheral physiological arousal as the difference in magnitude ΔSCL) of the median SCL response to Lab-TAB probes from the median SCL response during the first 10 sec of each probe (baseline). Multiple measures have widely been used to investigate EDA (mean, median, confidence intervals, square root mean transformation of SCL) [Lydon et al., 2016]. We chose to compute change in SCL using the median1 to reduce the influence of outliers and exhibit a better representation of the overall physiological response.
Statistical Analysis
To evaluate if the toddlers entered the tasks with inherent differences in basal arousal level, preliminary analyses evaluated the effects of diagnosis and condition on the magnitude of SCL at baseline using linear mixed model (LMM) analysis. Furthermore, to examine the change of magnitude of SCL from baseline within each diagnostic group and condition, we compared the magnitude of ΔSCL to zero using one-sample t-tests.
To test our primary hypotheses, an LMM approach was used to examine the effects of emotion inducing condition (Anger, Joy, and Fear) and diagnosis (ASD, TD) on ΔSCL. The task only required short verbal directions from the examiner to help transition between Lab-TAB probes; however, the groups differed in language skills, therefore an additional LMM analysis with verbal developmental quotient (VDQ) as a covariate was conducted to confirm that verbal ability was not linked to ΔSCL. Finally, we examined whether the magnitude of observed ΔSCL related to behavioral intensity of emotional expression as well as severity of autism symptoms measured by the ADOS-T in each condition in the ASD group. The analysis was conducted using Pearson’s r correlation coefficients.
Results
Preliminary Analyses
Magnitude of SCL at baseline.
A condition × diagnosis LLM analysis on SCL at baseline indicated no effect of diagnosis (F[1,71] = 2.83, P = 0.097, d = 0.39, 95% CI [−0.01, 0.86]; MeanASD = 6.80, SD = 8.90, MeanTD = 3.93, SD = 3.87). There was a significant effect of condition (F = 30.98, P < 0.001, d = 1.31, 95% CI [0.80, 1.81]) but no significant diagnosis × condition interaction (F = 2.05, P = 0.132, d = 0.34, 95% CI [−0.13, 0.80]). Post hoc tests revealed a significant increase in SCL throughout the three conditions (from MeanANGER = 4.71 [SD = 7.26] to MeanJOY = 5.49 [SD = 7.07] to MeanFEAR = 6.45 [SD = 7.48] condition in this order) (all Ps < 0.001).
Magnitude of ΔSCL.
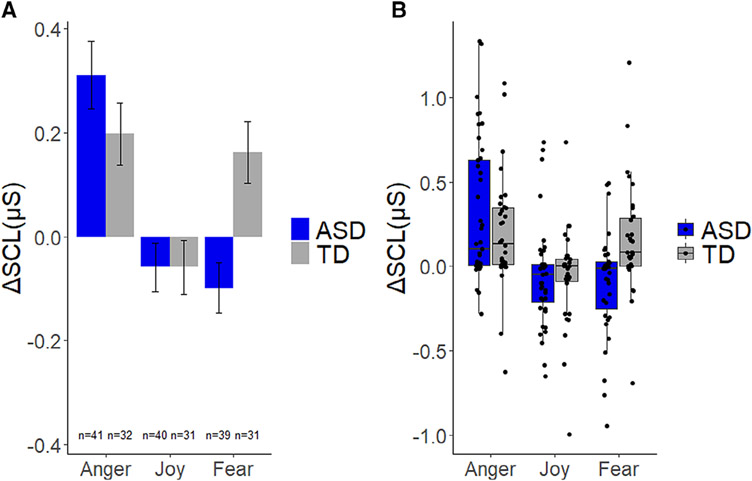
In the Anger condition, the analysis revealed that both groups exhibited increased ΔSCL from baseline (TD: t[31] = 3.31, P = 0.002, d = 0.59, 95% CI [0.07, 0.31]; ASD: t(40) = 4.76, P < 0.001, d = 0.74, 95% CI [0.15, 0.41]). In the Joy condition, neither TD (t[30] = 1.13, P = 0.267, d = 0.20, 95% CI [−0.15, 0.05]) nor ASD group showed statistically significant changes in ΔSCL from baseline (t[39] = 1.24, P = 0.223, d = 0.20, 95% CI [−0.04, 0.15]), Finally, in the Fear condition, toddlers with ASD showed a significant decrease in ΔSCL (t[38] = 2.04, P = 0.048, d = 0.33 95% CI [−0.20, −0.01]), unlike TD toddlers who exhibited a significant increase in ΔSCL (t[30] = 2.72, P = 0.011, d = 0.49, 95% CI [0.04, 0.28]).
Primary Analyses
Effect of diagnosis and emotion on ΔSCL.
A condition × diagnosis LLM analysis on ΔSCL indicated no effect of diagnosis (F[1,70] = 1.03, P = 0.314, d = 0.24, 95% CI [−0.22, 0.70]), but statistically significant effects of condition (F[2,137] = 17.91, P <0.001, d = 0.99, 95% CI [0.51, 1.48]) and a diagnosis × condition interaction (F = 5.74, P = 0.004, d = 0.56, 95% CI [0.09, 1.03]). Planned comparisons in the Anger condition showed that TD and ASD groups did not differ in ΔSCL (P = 0.206, d = 0.30). In the Joy condition, no statistical difference was found between groups (P = 0.996, d < 0.01). Finally, in the Fear condition, toddlers with ASD exhibited lower ΔSCL compared to TD controls (P = 0.001, d = 0.83) (Fig. 1, Panel A for overall means and Panel B for individual data points). To control for differences observed in VDQ between ASD and TD groups, a follow-up condition × group LLM analysis on ΔSCL was used with VDQ as a covariate. Results remained unchanged when VDQ score was included in the model. There was no effect of diagnosis (F[1,70] = 1.03, P = 0.314, d = 0.24, 95% CI [−0.22, 0.70]) or VDQ (F[1,70] = 0.02, P = 0.892, d = 0.03, 95% CI [−0.43, 0.49]), but a significant effect of condition (F = 17.91, P < 0.001, d = 0.99, 95% CI [0.51, 1.48]) and a significant diagnosis × condition interaction (F = 5.74, P = 0.004, d = 0.56, 95% CI [0.09, 1.03]).
Figure 1.
Overall means (Panel A) and medians, 25th and 75th percentiles as well as individual data points (Panel B) depicting changes of skin conductance level ΔSCL), compared to baseline, in micro-Siemens (μS) during anger, joy, and fear eliciting conditions for toddlers with ASD and typical development (TD).
Associations between ΔSCL and iEE.
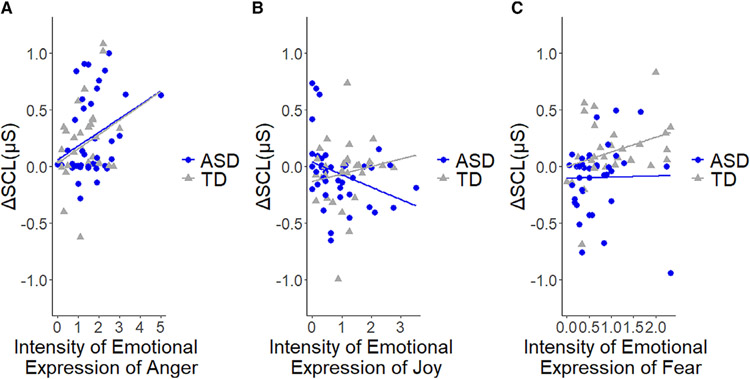
Analyses of the combined samples revealed a positive correlation between ΔSCL and iEE anger during the Anger condition (r[71] = .36, P = 0.002) as well as between ΔSCL and iEE fear (r[68] = 0.32, P = 0.007) during the Fear condition (Fig. 2). No statistically significant association between ΔSCL and iEE joy in the Joy condition was observed (r[69] = −0.14, P = 0.226) (Fig. 2). Although sample sizes decrease when examining associations of physiological and behavioral responses separately in each group, significant associations of ΔSCL and iEE were found in both Anger (r[39] = 0.36, P = 0.020) and Joy (r[38] = −0.32, P = 0.041) conditions in the ASD group but not in the Fear condition (r[37] = 0.02, P = 0.912). In the TD group, the correlation was significant in the Fear condition (r[29] = 0.42, P = 0.018), but not in the Anger (r[30] = 0.29, P = 0.103) and Joy (r[29] = 0.16, P = 0.402) conditions.
Figure 2.
Relative changes of skin conductance level (ΔSCL) during anger (Panel A), joy (Panel B), and fear (Panel C) eliciting conditions as a function of intensity of anger, joy, and fear expressions, respectively, in toddlers with ASD and typical development (TD).
Associations between ΔSCL and severity of autism.
Correlational analyses between ΔSCL and severity of autism symptoms (measured by ADOS-T, social affect, restricted and repetitive behaviors) in the ASD sample indicated no significant relationships in Anger, Joy, or Fear conditions (−0.24 < all rs < 0.16, .130 < all Ps < 0.860) (Table 2).
Table 2.
Association Between Changes in Skin Conductance Levels (ΔSCL) and Autism Severity in the ASD Group
| ΔSCL | ADOS SA score | ADOS RRB score |
|---|---|---|
| Anger | r(39) = −0.24, P = 0.130 | r(39) = 0.03, P = 0.860 |
| Joy | r(38) = 0.11, P = 0.483 | r(38) = 0.07, P = 0.676 |
| Fear | r(37) = 0.04, P = 0.821 | r(37) = 0.16, P = 0.321 |
Abbreviations: ADOS-2, Autism Diagnostic Observation Schedule, Second Edition (Total score of Toddler module); ASD, autism spectrum disorder; RRB, restricted and repetitive behaviors; SA, social affect.
Discussion
The present study examined an established physiological index of emotional responses to positively and negatively valenced events in toddlers with ASD. Emotional responses were quantified using changes in peripheral physiological arousal and observable facial and vocal expressions.
In response to probes aimed at eliciting anger or frustration, both the ASD and TD groups exhibited an increase in physiological arousal from baseline and there were no statistically significant differences between the ASD and TD groups in the magnitude of the increase. This is consistent with patterns observed at the behavioral level in a study of our research group [Macari et al., 2018]. In the combined samples, there was a significant positive correlation between the magnitude of changes in arousal and intensity of emotional expression. Together the studies suggest that in toddlerhood, responses to frustrating events are comparable between ASD and TD groups on the behavioral and physiological levels. The results obtained in our toddler sample are also somewhat consistent with a report in preschoolers with ASD who responded to frustrating events (e.g., locked box) with normative levels of intensity and duration of negatively valenced facial expressions [Jahromi et al., 2012].
Toddlers with ASD exhibited significantly lower physiological reactivity to novel and potentially threatening objects than toddlers with TD. This finding is consistent with the results of a study of our research group that examined the intensity of behavioral fear responses to the same events in toddlers with ASD compared to both their DD and TD peers [Macari et al., 2018]. Interestingly, while toddlers with TD exhibited increased physiological response to threat, the ASD group showed, paradoxically, a significant decrease in SCL from baseline when exposed to threatening nonsocial stimuli ΔSCLASD-TD = −0.26 μS, d = 0.83). These results are consistent with a report on school-age children with ASD who showed significantly weaker electrodermal reactivity to threat represented by images (e.g., sharks or anger faces) [Lydon et al., 2016], which may suggest hypo-responsivity to threatening stimuli in individuals with ASD [Blair, 1999]. The magnitude of physiological fear reactivity was positively correlated with intensity of observed behavioral fear responses in the overall sample of children, although not in the ASD group alone. The decreased arousal response of toddlers with ASD observed in this condition may reflect a number of possible factors. It may indicate interest in the novel stimuli, whereby the magnitude of decrease in arousal indexes depth of attentional engagement [Richards, Reynolds, & Courage, 2010]. It may also reflect a mild relaxation state that points to a failure to appraise the potentially threatening stimuli as such, suggesting risk for safety concerns. Alternatively, atypical attentiveness (e.g., in frequency, in duration, etc.) to the stimuli may be in play; other research has shown the complex associations existing between attentional factors and physiological arousal measured via EDA [Smith, Rockwell-Tischer, & Davidson, 1986]. Further research is needed to understand the underlying mechanisms of this atypical response to potentially threatening stimuli observed in toddlers with ASD.
Both ASD and TD groups showed no significant changes in physiological arousal from baseline in response to nonsocial probes aimed to elicit joy and showed comparable changes in SCL between groups. In the ASD group, intensity of emotional expression of joy was negatively associated with changes in SCL in the Joy condition. This pattern of results is consistent with that observed at the behavioral level in a study of our research group which also showed no overall differences between toddlers with and without ASD regarding expressivity of Joy in response to playful tasks [Macari et al., 2018]. With prior research demonstrating comparable decreases in electrodermal responses in older children playing with peers in the presence of animals [O’haire, McKenzie, Beck, & Slaughter, 2015], taken together our findings emphasize the benefit of capitalizing on incentives that do not solely involve adults to regulate arousal in toddlers with ASD.
Notably, in our study, intensity of physiological EDA responses to negatively valenced challenges was significantly correlated with intensity of behavioral emotional expression, which provides, to the best of our knowledge, the first evidence of congruency between behavioral and physiological responses to nonsocial emotional challenges in a sample of toddlers with and without ASD. More specifically, these results suggest that relative change in SCL observed in response to more naturalistic challenges is positively related to intensity of behavioral expression displayed by toddlers with and without ASD during anger- and fear- eliciting tasks. Less consistent patterns of correlations were observed when the analyses were conducted separately in each group, which might be due to diminished power to detect the effects in smaller samples and restricted ranges of scores after the groups were considered separately. Replication of these results in larger studies is warranted.
Although previous research has found mixed results regarding EDA at baseline in children with ASD (hypo, hyper, or typical SCL) [Lydon et al., 2016], our study revealed a trend, albeit not statistically significant, of elevated SCL prior to exposure to emotion-eliciting challenges in toddlers with ASD compared to their TD peers at 22 months of age. This resonates with the finding of elevated basal EDA reported by several other research groups examining older children with ASD [Cohen et al., 2015;Palkovitz & Wiesenfeld, 1980]. Further rigorous examination of physiological arousal before stimulation in young children with ASD is warranted in order to better understand the influence of basal physiological levels in children with ASD over the course of everyday activities.
Changes in EDA did not correlate with autism severity in our sample; however, atypical physiological response patterns may still contribute to the development of heterogeneous autism phenotypes later in life [Fenning et al., 2017] and forecast subsequent emotional difficulties highly comorbid with ASD. This is consistent with fearlessness theory and a body of work indicating low physiological reactivity (among which are attenuated electrodermal responses) being a risk for conduct problems [Cappadocia, Desrocher, Pepler, & Schroeder, 2009; Raine, Venables, & Mednick, 1997], especially in children [Beauchaine, 2001;Erath, El-Sheikh, Hinnant, & Cummings, 2011;Erath, El-Sheikh, & Mark Cummings, 2009]. Although the nature of these associations in very young children with ASD remains to be determined, recent work in children with ASD between 4 and 11 years of age showed that low EDA responses during compliance-oriented tasks predict higher child externalizing problems, possibly moderated by parental regulatory support and scaffolding [Baker et al., 2018]. Being able to predict comorbidities and developmental outcomes using physiological arousal combined with other measures may elucidate early mechanistic features of emotional dysregulation and facilitate downstream improvements (i.e., primary prevention, early intervention) for many individuals with ASD. Promising work investigating sympathetic-parasympathetic interaction by measuring heart rate variability and EDA to a pen-and-paper challenging task has indeed revealed the possible existence of two distinct psychophysiological pathways linked to heightened externalizing behavior problems in children with autism between 6 and 10 years of age [Fenning et al., 2019]. We are currently following our toddler sample into preschool age and will be able to evaluate developmental contingencies between physiological arousal and comorbid conditions in the future.
As previously reported in older children with ASD [Fenning et al., 2017;Fenning et al., 2019;Goodwin, 2016;Schupak, Parasher, & Zipp, 2016], the current results demonstrate that changes in ambulatory EDA response is a feasible and valid measure of peripheral physiological arousal during more naturalistic situations in very young ASD and TD children. Capitalizing on the accessibility and usability of newer mobile sensors (able to measure multiple physiological markers simultaneously) to concurrently examine larger groups of children would provide this more complete picture and help replicate, support, and extend our study with its relatively large but population-modest sample size. Importantly, the current findings also highlight that emotional experience in toddlers with ASD indexed by EDA is influenced by situational valence, thus is context dependent. In certain emotion-eliciting situations (Anger and Joy condition), toddlers with ASD showed a somewhat typical arousal response; however, in other contexts (Fear condition), they showed attenuated arousal responses which may partially explain inconsistencies in prior reports. While these results do not suggest that toddlers with ASD are always “dysregulated” or “up-regulated,” they do suggest potential differential regulation.
Limitations
First, some effects missed statistical significance (e.g., magnitude of the increase of EDA responses in the Anger condition slightly higher in the ASD group compared to the TD group ΔSCL-AngerASD-TD = 0.11 μS, d = 0.30), raising the potential that the study was under-powered to detect certain effects. Replication of these findings in larger samples is warranted. Secondly, the emotional challenges used in the current study were largely nonsocial (i.e., involved the use of objects and did not require participants to interact directly with examiners). This methodology and the use of non-social stimuli were selected to avoid confounding effects between social and emotional vulnerabilities in the ASD sample. However, given that both valence and the social nature of emotions can influence neural substrates (anterior cingulate, nucleus accumbens, orbitofrontal cortex, and amygdala) of emotional processing [Britton et al., 2006], further research is needed to examine how the social nature of real life challenges may affect physiological arousal in toddlers with ASD. Such an approach would assist in determining if the atypical sympathetic responses in ASD observed here are domain-specific (i.e., elicited by nonsocial emotional triggers) or domain-general (i.e., elicited by both nonsocial and social triggers). Thirdly, in our prior work, behavioral emotional expression patterns differentiated toddlers with ASD from both DD and TD controls [Macari et al., 2018]. The present study did not include toddlers with DD, and thus specificity of the observed findings to ASD remains to be determined at the physiological level. However, our findings are potentially not driven by language ability as our results remained unchanged after controlling for verbal ability level.
Future Directions
As with social symptoms, emotional atypicalities are subject to developmental modifications through growth and external experiences and therefore require examination through prospective longitudinal studies. Furthermore, since EDA solely reflects activity of the sympathetic nervous system, it would be beneficial to simultaneously assess the parasympathetic branch given its role in emotion regulation and assistance in recovering from stressful events [Langley, 1921]. This could be achieved by combining more than a few physiological indicators such as heart rate variability or blood pressure [Kushki et al., 2013;Levine et al., 2012;Schupak et al., 2016] to get a more complete picture of the full autonomic response profiles present in toddlers with ASD. Given the association between physiological and behavioral response observed during the Joy condition in toddlers with ASD in the current study, future investigations of physiological reactivity to joyful situations in ASD would benefit from utilization of toys or specific interests thought to trigger positive emotions.
Conclusions
Peripheral physiological responses to emotional challenges in toddlers with ASD are modulated by contextual factors, consistent with observations of behavioral emotional expression [Macari et al., 2018]. Our findings collectively reveal converging behavioral and physiological evidence for an attenuated response to threatening stimuli in toddlers with ASD. Future studies will be needed to understand the potential cognitive, attentional, or other factors underlying this response. Considering the substantial influence physiological arousal has on perceptual, cognitive, affective, and social development [Jahromi et al., 2012;Mather & Sutherland, 2011;O’haire et al., 2015], examining physiological responses to a variety of more naturalistic situations early on in children with ASD is of considerable theoretical and clinical importance. The identified atypical emotional reactivity profiles may put toddlers with ASD at risk for a range of comorbid psychopathologies [Lengua, 2003], while the typical physiological response found in response to playful nonsocial events suggests a possible point of resiliency, considering the protective influences of positive emotionality on early development and social adjustment in the general population [Lengua, 2003].
Acknowledgments
The study was supported by the National Institute of Mental Health R01 MH111652 and R01 MH100182 (to K. C.). The authors wish to express their sincere appreciation to the families and children for their participation in this study. Finally, the authors would like to thank all three-dedicated peer-reviewers who contributed to enriching this manuscript.
Footnotes
Conflict of Interest
Angelina Vernetti, Suzanne Macari, Laura Boccanfuso, Katarzyna Chawarska, Finola Kane-Grade, Anna Milgramm, Emily Hilton, and Perrine Heymann report no biomedical financial interests or potential conflicts of interest. Frederick Shic is a Consultant for Roche and Janssen. Matthew Goodwin is a Scientific Advisory Board member of Affectiva and a Consultant for Janssen.
An additional analysis using the mean did not affect the omnibus significance levels reported for median.
Contributor Information
Angelina Vernetti, Child Study Center, Yale University School of Medicine, New Haven, Connecticut, USA.
Frederick Shic, Child Study Center, Yale University School of Medicine, New Haven, Connecticut, USA; Seattle Children’s Research Institute, Seattle, Washington, USA; Division of General Pediatrics, University of Washington School of Medicine, Seattle, Washington, USA.
Laura Boccanfuso, Vän Robotics, Columbia, South Carolina, USA.
Suzanne Macari, Child Study Center, Yale University School of Medicine, New Haven, Connecticut, USA.
Finola Kane-Grade, Division of Developmental Medicine, Boston Children’s Hospital, Boston, Massachusetts, USA.
Anna Milgramm, Center for Autism and Related Disabilities, University at Albany, SUNY, New York City, New York, USA.
Emily Hilton, Department of Psychology, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Perrine Heymann, Early Childhood Behavior Lab, Florida International University, Miami, Florida, USA.
Matthew S Goodwin, Department of Health Sciences, Bouvé College of Health Sciences, Northeastern University, Boston, Massachusetts, USA.
Katarzyna Chawarska, Child Study Center, Yale University School of Medicine, New Haven, Connecticut, USA.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Washington DC: American Psychiatric Association. [Google Scholar]
- Baker JK, Fenning RM, Erath SA, Baucom BR, Moffitt J, & Howland MA (2018). Sympathetic under-arousal and externalizing behavior problems in children with autism spectrum disorder. Journal of Abnormal Child Psychology, 46(4), 895–906. 10.1007/s10802-017-0332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13(2), 183–214. 10.1017/s0954579401002012 [DOI] [PubMed] [Google Scholar]
- Benedek M, & Kaernbach C (2010). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods, 190(1), 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (1999). Psychophysiological responsiveness to the distress of others in children with autism. Personality and Individual Differences, 26(3), 477–485. [Google Scholar]
- Boucsein W (2012). Electrodermal activity. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- Bradley MM, & Lang PJ (2000). Measuring emotion: Behavior, feeling, and physiology. Cognitive Neuroscience of Emotion, 25, 49–59. [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, & Liberzon I (2006). Neural correlates of social and nonsocial emotions: An fMRI study. NeuroImage, 31(1), 397–409. [DOI] [PubMed] [Google Scholar]
- Cappadocia MC, Desrocher M, Pepler D, & Schroeder JH (2009). Contextualizing the neurobiology of conduct disorder in an emotion dysregulation framework. Clinical Psychology Review, 29(6), 506–518. 10.1016/j.cpr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, & Volkmar F (2009). A prospective study of toddlers with ASD: Short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry, 50(10), 1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Masyn K, Mastergeorge A, & Hessl D (2015). Psychophysiological responses to emotional stimuli in children and adolescents with autism and fragile X syndrome. Journal of Clinical Child & Adolescent Psychology, 44(2), 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AP, Steffgen G, & Samson AC (2017). Expressive incoherence and alexithymia in autism Spectrum disorder. Journal of Autism and Developmental Disorders, 47(6), 1659–1672. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, & Filion DL (2000). The electrodermal system. In: Tassinary LG, Berntson GG, Cacioppo JT (Eds). Handbook Of Psychophysiology (pp. 200–223). Cambridge: Cambride University Press. [Google Scholar]
- De Pauw SSW, Mervielde I, Van Leeuwen KG, & De Clercq BJ (2011). How temperament and personality contribute to the maladjustment of children with autism. Journal of Autism and Developmental Disorders, 41(2), 196–212. 10.1007/s10803-010-1043-6 [DOI] [PubMed] [Google Scholar]
- Ekman P (1992). Are there basic emotions? Psychological Review, 99(3), 550–553. 10.1037/0033-295X.99.3.550 [DOI] [PubMed] [Google Scholar]
- Erath SA, El-Sheikh M, Hinnant JB, & Cummings EM (2011). Skin conductance level reactivity moderates the association between harsh parenting and growth in child externalizing behavior. Developmental Psychology, 47(3), 693–706. 10.1037/a0021909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erath SA, El-Sheikh M, & Mark Cummings E (2009). Harsh parenting and child externalizing behavior: Skin conductance level reactivity as a moderator. Child Development, 80 (2), 578–592. 10.1111/j.1467-8624.2009.01280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenning RM, Baker JK, Baucom BR, Erath SA, Howland MA, & Moffitt J (2017). Electrodermal variability and symptom severity in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(4), 1062–1072. [DOI] [PubMed] [Google Scholar]
- Fenning RM, Erath SA, Baker JK, Messinger DS, Moffitt J, Baucom BR, & Kaeppler AK (2019). Sympathetic-parasympathetic interaction and externalizing problems in children with autism Spectrum disorder. Autism Research, 12(12), 1805–1816. 10.1002/aur.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA (1989). Psychophysiological correlates of emotional reactivity during the first year of life. Developmental Psychology, 25(3), 364–372. 10.1037/0012-1649.25.3.364 [DOI] [Google Scholar]
- Gadow KD, DeVincent C, & Schneider J (2008). Predictors of psychiatric symptoms in children with an autism spectrum disorder. Journal of Autism and Developmental Disorders, 38 (9), 1710–1720. [DOI] [PubMed] [Google Scholar]
- Gagne JR, Van Hulle CA, Aksan N, Essex MJ, & Goldsmith H (2011). Deriving childhood temperament measures from emotion-eliciting behavioral episodes: Scale construction and initial validation. Psychological Assessment, 23 (2), 337–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, & Szatmari P (2009). Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. Journal of Abnormal Child Psychology, 37(1), 59–78. [DOI] [PubMed] [Google Scholar]
- Garon N, Zwaigenbaum L, Bryson S, Smith IM, Brian J, Roncadin C, … Roberts W (2016). Temperament and its association with autism symptoms in a high-risk population. Journal of Abnormal Child Psychology, 44(4), 757–769. [DOI] [PubMed] [Google Scholar]
- Goldsmith H, & Rothbart MK (1999). The laboratory temperament assessment battery (Locomotor version). Madison: University of Wisconsin-Madison. [Google Scholar]
- Goodwin MS (2016). 28.2 Laboratory and home-based assessment of electrodermal activity in individuals with autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 55(10), S301–S302. [Google Scholar]
- Green SA, Ben-Sasson A, Soto TW, & Carter AS (2012). Anxiety and sensory over-responsivity in toddlers with autism spectrum disorders: Bidirectional effects across time. Journal of Autism and Developmental Disorders, 42(6), 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie W, Swineford LB, Nottke C, & Wetherby AM (2013). Early diagnosis of autism spectrum disorder: Stability and change in clinical diagnosis and symptom presentation. Journal of Child Psychology and Psychiatry, 54(5), 582–590. 10.1111/jcpp.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney JN, & Euse FJ (1976). Skin conductance and heart rate responses to neutral, positive, and negative imagery: Implications for convert behavior therapy procedures. Behavior Therapy, 7(4), 494–503. [Google Scholar]
- Hirstein W, Iversen P, & Ramachandran V (2001). Autonomic responses of autistic children to people and objects. Proceedings of the Royal Society of London B: Biological Sciences, 268(1479), 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE, Huebner RR, Risser D, & Dougherty L (1980). The young infant’s ability to produce discrete emotion expressions. Developmental Psychology, 16(2), 132–140. [Google Scholar]
- Jahromi LB, Meek SE, & Ober-Reynolds S (2012). Emotion regulation in the context of frustration in children with high functioning autism and their typical peers. Journal of Child Psychology and Psychiatry, 53(12), 1250–1258. [DOI] [PubMed] [Google Scholar]
- Kaartinen M, Puura K, Mäkelä T, Rannisto M, Lemponen R, Helminen M, … Hietanen JK (2012). Autonomic arousal to direct gaze correlates with social impairments among children with ASD. Journal of Autism and Developmental Disorders, 42(9), 1917–1927. [DOI] [PubMed] [Google Scholar]
- Kim SH, Macari S, Koller J, & Chawarska K (2015). Examining the phenotypic heterogeneity of early autism Spectrum disorder: Subtypes and short-term outcomes. Journal of Child Psychology and Psychiatry, 57(1), 93–102. 10.1111/jcpp.12448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, & Hellhammer DH (1993). The ‘Trier social stress test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Kleckner IR, Jones RM, Wilder-Smith O, Wormwood JB, Akcakaya M, Quigley KS, … Goodwin MS (2017). Simple, transparent, and flexible automated quality assessment procedures for ambulatory Electrodermal activity data. IEEE Transactions on Biomedical Engineering. 10.1109/tbme.2017.2758643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. [DOI] [PubMed] [Google Scholar]
- Kushki A, Drumm E, Mobarak MP, Tanel N, Dupuis A, Chau T, & Anagnostou E (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS One, 8(4), e59730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylliäinen A, & Hietanen JK (2006). Skin conductance responses to another person’s gaze in children with autism. Journal of Autism and Developmental Disorders, 36(4), 517–525. [DOI] [PubMed] [Google Scholar]
- Langley JN (1921). The autonomic nervous system. Cambridge, England: W. Heffer & Sons, Limited. [Google Scholar]
- Lazarus RS (1991). Emotion and adaptation. Oxford, England: Oxford University Press on Demand. [Google Scholar]
- Lengua LJ (2003). Associations among emotionality, self-regulation, adjustment problems, and positive adjustment in middle childhood. Journal of Applied Developmental Psychology, 24(5), 595–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TP, Sheinkopf SJ, Pescosolido M, Rodino A, Elia G, & Lester B (2012). Physiologic arousal to social stress in children with autism spectrum disorders: A pilot study. Research in Autism Spectrum Disorders, 6(1), 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, … Lainhart JE (2006). Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36(7), 849–861. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles, CA: Western Psychological Corporation. [Google Scholar]
- Louwerse A, Van Der Geest J, Tulen J, van der Ende J, Van Gool A, Verhulst F, & Greaves-Lord K (2013). Effects of eye gaze directions of facial images on looking behaviour and autonomic responses in adolescents with autism spectrum disorders. Research in Autism Spectrum Disorders, 7(9), 1043–1053. [Google Scholar]
- Lydon S, Healy O, Reed P, Mulhern T, Hughes BM, & Goodwin MS (2016). A systematic review of physiological reactivity to stimuli in autism. Developmental Neurorehabilitation, 19(6), 335–355. [DOI] [PubMed] [Google Scholar]
- Macari S, DiNicola L, Kane-Grade F, Prince E, Vernetti A, Powell K, … Chawarska K (2018). Emotional expressiveness in toddlers with autism Spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry., 57, 828–836.e2. 10.1016/j.jaac.2018.07.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macari S, Koller J, Campbell DJ, & Chawarska K (2017). Temperamental markers in toddlers with autism spectrum disorder. Journal of Child Psychology and Psychiatry, 58(7), 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Sutherland MR (2011). Arousal-biased competition in perception and memory. Perspectives on Psychological Science, 6(2), 114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion, 5(2), 175–190. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, & White SW (2014). Emotion regulation: Concepts & practice in autism spectrum disorder. Child and Adolescent Psychiatric Clinics of North America, 23(1), 15–24. 10.1016/j.chc.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Hessl D, Macari SL, Ozonoff S, Green C, & Rogers SJ (2014). Electrodermal and behavioral responses of children with autism spectrum disorders to sensory and repetitive stimuli. Autism Research, 7(4), 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning [measurement instrument]. Circle Pines, MN: AGS. [Google Scholar]
- Naber FB, Swinkels SH, Buitelaar JK, Bakermans-Kranenburg MJ, van IJzendoorn MH, Dietz C, … van Engeland H (2007). Attachment in toddlers with autism and other developmental disorders. Journal of Autism and Developmental Disorders, 37(6), 1123–1138. [DOI] [PubMed] [Google Scholar]
- Neuhaus E, Bernier RA, & Beauchaine TP (2015). Electrodermal response to reward and non-reward among children with autism. Autism Research, 8(4), 357–370. [DOI] [PubMed] [Google Scholar]
- O’haire ME, McKenzie SJ, Beck AM, & Slaughter V (2015). Animals may act as social buffers: Skin conductance arousal in children with autism spectrum disorder in a social context. Developmental Psychobiology, 57(5), 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovitz RJ, & Wiesenfeld AR (1980). Differential autonomic responses of autistic and normal children. Journal of Autism and Developmental Disorders, 10(3), 347–360. [DOI] [PubMed] [Google Scholar]
- Planalp EM, Van Hulle C, Gagne JR, & Goldsmith HH (2017). The infant version of the laboratory temperament assessment battery (lab-TAB): Measurement properties and implications for concepts of temperament. Frontiers in Psychology, 8(846), 885–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince EB, Kim ES, Wall CA, Gisin E, Goodwin MS, Simmons ES, … Shic F (2017). The relationship between autism symptoms and arousal level in toddlers with autism spectrum disorder, as measured by electrodermal activity. Autism, 21(4), 504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Venables PH, & Mednick SA (1997). Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius child health project. Journal of the American Academy of Child and Adolescent Psychiatry, 36(10), 1457–1464. 10.1097/00004583-199710000-00029 [DOI] [PubMed] [Google Scholar]
- Richards JE, Reynolds GD, & Courage ML (2010). The neural bases of infant attention. Current Directions in Psychological Science, 19(1), 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar F, Baird G, Chandler S, Tseng E, O’sullivan T, Howlin P, … Simonoff E (2015). Co-occurring psychiatric disorders in preschool and elementary school-aged children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(8), 2283–2294. [DOI] [PubMed] [Google Scholar]
- Scherr JF, Hogan AL, Hatton D, & Roberts JE (2017). Stranger fear and early risk for social anxiety in preschoolers with fragile X syndrome contrasted to autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(12), 3741–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I, Regenbogen C, Kohn N, Zepf FD, Bubenzer-Busch S, Schneider F, … Habel U (2015). Reduced responsiveness to social provocation in autism spectrum disorder. Autism Research, 8(3), 297–306. [DOI] [PubMed] [Google Scholar]
- Schupak BM, Parasher RK, & Zipp GP (2016). Reliability of electrodermal activity: Quantifying sensory processing in children with autism. American Journal of Occupational Therapy, 70(6), 7006220030p7006220031–7006220030p7006220036. [DOI] [PubMed] [Google Scholar]
- Shalom DB, Mostofsky S, Hazlett R, Goldberg M, Landa R, Faran Y, … Hoehn-Saric R (2006). Normal physiological emotions but differences in expression of conscious feelings in children with high-functioning autism. Journal of Autism and Developmental Disorders, 36(3), 395–400. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. [DOI] [PubMed] [Google Scholar]
- Smith BD, Rockwell-Tischer S, & Davidson R (1986). Extraversion and arousal: Effects of attentional conditions on electrodermal activity. Personality and Individual Differences, 7 (3), 293–303. [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV, Harrison PL, & Doll EA (1984). Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Stifter CA, Fox NA, & Porges SW (1989). Facial expressivity and vagal tone in 5-and 10-month-old infants. Infant Behavior and Development, 12(2), 127–137. [Google Scholar]
- Storm H (2000). Skin conductance and the stress response from heel stick in preterm infants. Archives of Disease in Childhood-Fetal and Neonatal Edition, 83(2), F143–F147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Mazefsky CA, Dichter GS, Chiu PH, Richey JA, & Ollendick TH (2014). Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: Understanding anxiety in autism spectrum disorder. International Journal of Developmental Neuroscience, 39, 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, & Gadow KD (2010). Exploring the nature and function of anxiety in youth with autism spectrum disorders. Clinical Psychology: Science and Practice, 17(4), 281–292. [Google Scholar]