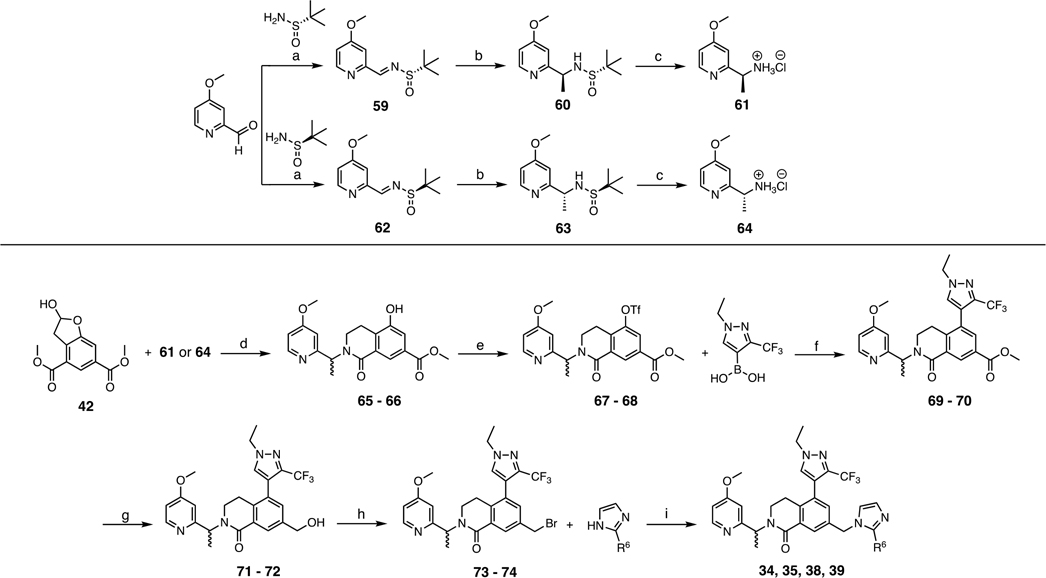
Scheme 4.
Synthesis of Compounds 34, 35, 38, and 39a
aR6 is defined in Table 4. Conditions: (a) (S)- or (R)-2-methylpropane-2-sulfinamide, Cs2CO3, CH2Cl2, rt, overnight, 65%; (b) MeMgBr (3.4 M 2-MeTHF), THF, −78 °C, 84–92%; (c) HCl (4 M 1,4-dioxane), THF, 94%—quant.; (d) chiral amine, i-Pr2NEt, NaBH(OAc)3, CH2Cl2, rt, overnight, then 1,4-dioxane, 110 °C, 84%; (e) phenyl triflimide, i-Pr2NEt, THF/CH2Cl2, rt, overnight, 93%; (f) (1-ethyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)boronic acid, Pd(PPh3)4, Na2CO3, 1,4-dioxane/H2O, 80 °C, 96%; (g) LiBH4 (2 M THF) or LiBHEt3 (1 M THF), THF, 0 °C, 1 h, 72–85%; (h) CH3SO2Cl, i-Pr2NEt, CH2Cl2, 0 °C, then LiBr, THF, reflux, 53% or PBr3, CH2Cl2, 0 °C, 98%; (i) R6-imidazole, MeCN, 50 °C, overnight, 73%.