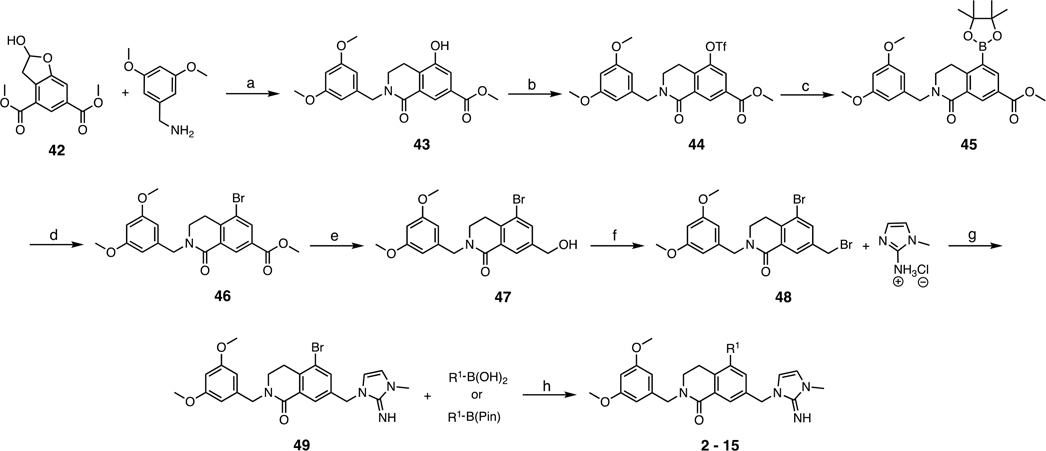
Scheme 1.
Synthesis of Imidazole-Imine Series Compounds 2–15a
aR1 is defined in Table 1. Conditions: (a) 3,5-dimethoxybenzylamine, NaBH(OAc)3, CH2Cl2, rt, overnight, then 1,4-dioxane, 110 °C, 99%; (b) phenyl triflimide, i-Pr2NEt, THF/CH2Cl2, rt, overnight, 79%; (c) B2Pin2, KOAc, PdCl2(dppf)·CH2Cl2, 1,4-dioxane, 100 °C overnight, 99%; (d) CuBr2, MeOH/H2O, 80 °C, 90%; (e) LiBHEt3 (1 M THF), THF, 0 °C, 1 h, 41%; (f) PBr3, CH2Cl2, 0 °C, 42%; (g) 1-methyl-1H-imidazol-2-amine hydrochloride, i-Pr2NEt, MeCN, 60 °C, overnight, quant.; (h) R1–B(OH)2 or R1–B(Pin), PdCl2(dppf)·CH2Cl2, K2CO3, 1,4-dioxane/H2O, 80 °C, overnight, 20–70%.