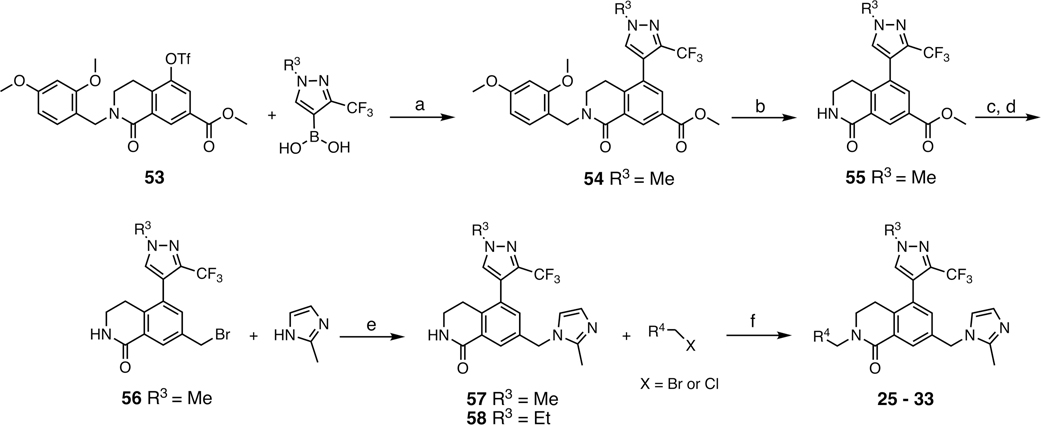
Scheme 3.
Synthesis of Pyridyl Series Compounds 25–33a
aR3 and R4 are defined in Table 3. Synthesis of 58 was reported in Scheme S1. Conditions: (a) R3-boronic acid, PdCl2(dppf)·CH2Cl2, K2CO3, 1,4-dioxane/H2O, 90 °C, overnight, 90%; (b) TFA, anisole, CH2Cl2, rt, overnight, 87%; (c) LiBHEt3 (1 M THF), THF, 0 °C, 1 h, quant.; (d) PBr3, CH2Cl2, 0 °C, quant.; (e) 2-methyl-1H-imidazole, MeCN, 50 °C, overnight, 70%; (f) R4-pyridylmethyl halide, NaH, DMF, 0 °C, 2 h, 25–71%.