Abstract
Vulnerability to compulsive drug use stems from dysregulated activity within the neural networks that underlie reward and executive functions. Empirical evidence suggests that a) attributing high motivational salience to drug-related stimuli leads to compulsive drug seeking and b) cognitive control deficits lead to compulsive drug taking. Noninvasive neuroimaging techniques enable brain activity monitoring during affective and cognitive processing and are paving the way to precision medicine for substance use disorders. Identifying robust neuromarkers of affective and cognitive dysregulation would allow clinicians to personalize treatments by targeting individual psychophysiological vulnerabilities. However, methodological choices have biased the field toward experimental paradigms that cannot optimally assess individual differences in the motivational salience of drug-related cues and in the ability to control drug-related decisions, choices which have hindered the identification of clinically relevant neuromarkers. Here, we show that once these shortcomings are amended, replicable neuromarkers of the tendency to attribute motivational salience to drug-related cues and the ability to control drug-related decisions emerge. While we use tobacco use disorder as a model, we also show that the methodological issues highlighted here are relevant to other disorders characterized by maladaptive appetitive behaviors.
Keywords: Biomarkers, Event-Related Potential, Motivational Salience, Cognitive Control, Drug Self-Administration
1. Introduction: From understanding the neurobiological underpinnings of SUDs to identifying clinically relevant neuromarkers
Substance use disorders (SUDs) are characterized by compulsive drug seeking and reduced control over drug taking [1]. Neurobiological models attribute these behaviors to dysregulated activity within the neural networks supporting reward and executive control such that excessive incentive salience attributed to drug-related cues (i.e., stimuli associated with the drug and its effects) increases vulnerability to compulsive drug seeking [2], while deficits in executive control reduce the ability to control drug taking [3].
Incentive salience refers to the motivational properties that make rewards (e.g., food, water, sex) and the stimuli predicting them (i.e., reward-related cues) attractive. Stimuli with high incentive salience capture attention, activate affective states, and motivate reward-seeking behaviors [4]. By efficiently attributing incentive salience to cues predicting rewards, organisms can prioritize and modify their consummatory behaviors to changes in the environment [5]. The information about a stimulus’ incentive salience is coded in the brain’s mesocorticolimbic dopamine systems [6] and it spreads through striatocortical brain reward pathways to modulate affective responses and appetitive behaviors [7–10]. Repeated supraphysiological drug-induced dopamine bursts can sensitize the reward dopamine systems of vulnerable individuals, making them hyperresponsive to drug-related cues [11]. Once drug-related cues acquire high levels of incentive salience, they trigger strong cravings and, ultimately, compulsive drug seeking, which leads to relapse [12].
In addition to attributing high incentive salience to drug-related cues, individuals with SUDs also have difficulties controlling drug cravings and inhibiting drug taking [13,14]. According to the impaired response inhibition and salience attribution (iRISA) model [3,15], compulsive drug taking stems from deficits in executive control. Executive control refers to the ability to select actions on the basis of internal plans and goals [16] and it is supported by a network of regions in the prefrontal cortex. On average, the network of prefrontal brain regions that supports executive controls is less active in individuals with SUDs than in healthy controls [17].
Identifying neuromarkers of these putative psychophysiological mechanisms using event-related potentials (ERPs, a direct measure of brain activity with high temporal resolution [18]) and functional magnetic resonance imaging (fMRI, an indirect measure of brain activity with high spatial resolution [19]) has high clinical relevance and it is an active area of research [20–23]. However, few neuromarkers with clinical utility have been identified so far. While some of the obstacles that delayed progress are common across biological psychiatry [24], others might be due to methodological choices that have biased the field of addiction neuroscience towards experimental paradigms that are not ideal for assessing SUDs’ psychophysiological underpinnings.
Below, we outlined what we believe to be the most relevant methodological shortcomings of the neurobehavioral assessments commonly used to assess the extent to which individuals with SUD attribute high incentive salience to drug-related cues or struggle with controlling drug-related decisions, and how these shortcomings can be amended. We showed that when these methodological issues are addressed, replicable neuromarkers with predictive validity that can be deployed in clinical settings emerge. We used nicotine use disorder as a model, but the methodological aspects that we highlighted are relevant to other substance use disorders and to excessive eating, the ultimate cause of obesity.
2. Recommendation 1: To identify neuromarkers of drug-related cues’ motivational salience, neuroaffective responses to non-drug-related motivationally relevant stimuli must be considered.
Neurobiological models of SUDs converge in indicating that attributing high incentive salience to drug-related cues increases vulnerability to compulsive drug seeking [15]. To study the neurophysiological underpinnings of the tendency to attribute incentive salience to drug-related cues in humans, addiction neuroscientists designed the cue-reactivity paradigm. In one classic cue-reactivity paradigm, neurophysiological responses are measured while study participants look at a slideshow in which drug-related and neutral images are presented. The difference in the neurophysiological responses evoked by drug-related and neutral cues is interpreted as a measure of the incentive salience attributed to cues.
Figure 1 illustrates why comparing brain responses evoked by drug-related cues to those evoked by neutral stimuli alone does not allow for strong conclusions about the motivational salience individuals attribute to drug-related cues [25,26]. Panel A shows the hypothetical brain responses of two smokers during a cue-reactivity task in which cigarette-related and neutral images are presented. Both smokers respond similarly to the neutral cues, but Smoker 1 reacts more strongly than Smoker 2 to the drug-related cues. These findings do not warrant concluding that cigarette-related cues are more salient for Smoker 1 than for Smoker 2 because while the brain responses evoked by the neutral stimuli provide the starting point of the hypothetical scale measuring motivational salience, the responses evoked by cigarette cues do not necessarily represent the top of that hypothetical reactivity scale. To more accurately determine the full range of the scale, responses to non-drug-related motivationally relevant pleasant (and unpleasant) stimuli) must also be measured. Panel B shows that Smoker 1 reacts more strongly to non-drug-related pleasant stimuli than to cigarette cues while Smoker 2 has the opposite reactivity profile, higher responses to drug-related cues than to pleasant stimuli, a condition we refer to as “increased substance cue reactivity” (SCR+). Observing these results would indicate that Smoker 2 attributes higher salience to drug-related cues than Smoker 1, who exhibits “reduced substance cue reactivity” (SCR−). This approach allows us to gauge the motivational relevance of drug-related cues by measuring neuroaffective responses to non-drug-related stimuli varying in both valence and motivational relevance while providing multiple active control conditions to estimate the reliability of the results.
Figure 1: Cue reactivity can be misleading.
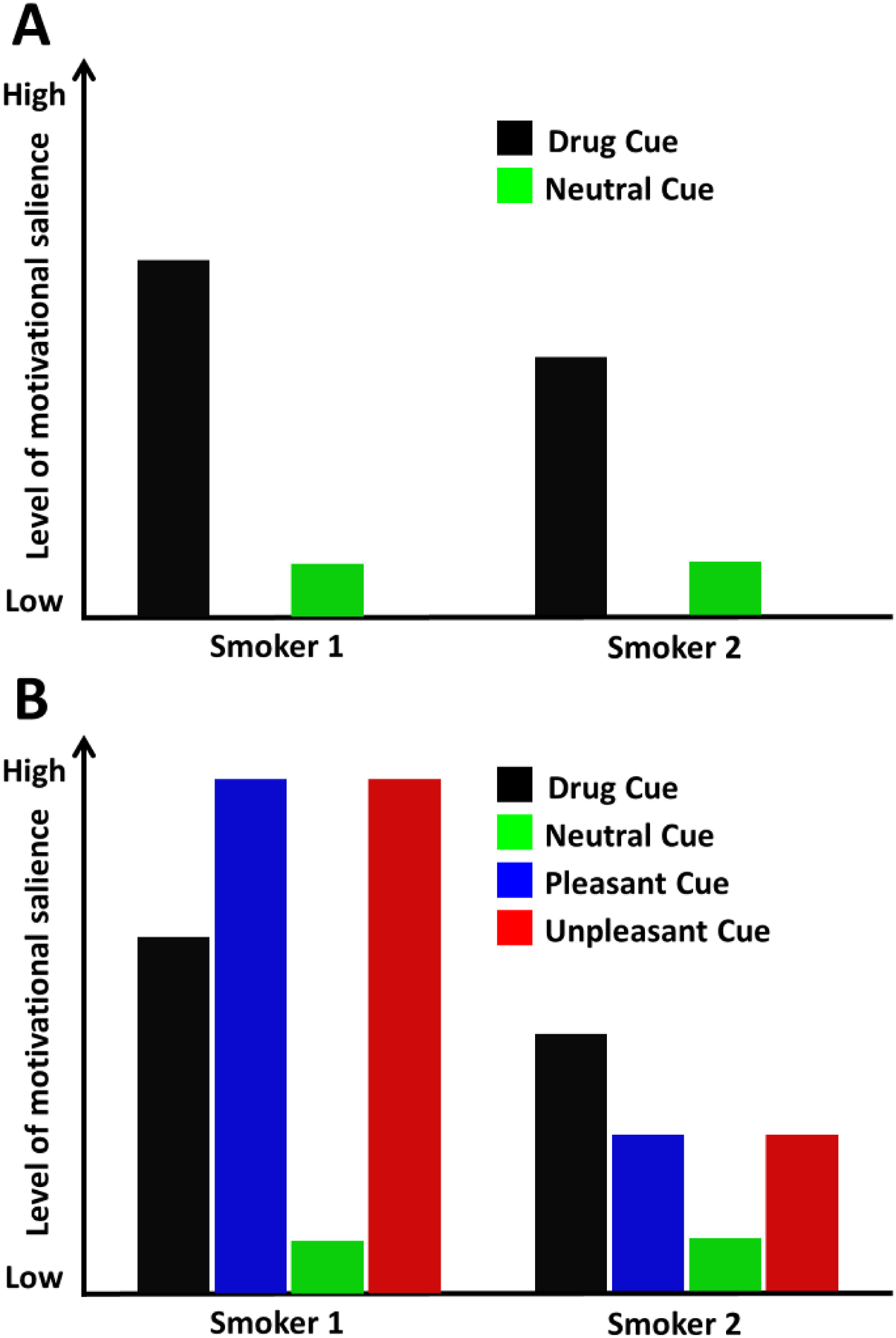
Without measuring responses to non-drug-related motivationally relevant stimuli, reactivity to drug-related cues cannot be interpreted. Panel A shows the hypothetical neurophysiological responses evoked by drug-related and neutral stimuli in two smokers. If we calculate “cue reactivity” as the difference cue minus neutral, it seems that “Smoker 1” has stronger responses to drug cues than “Smoker 2”. However, Panel B shows that Smoker 1 reacts more to non-drug-related motivationally relevant stimuli than to drug-related cues, while Smoker 2 reacts more to drug-related cues than to pleasant stimuli. Thus, non-drug-related motivationally relevant stimuli should be considered a more appropriate comparison for determining drug cue reactivity.
2.1. Example: Individual differences in reactivity to drug-related and pleasant cues predict vulnerability to drug use.
While Figure 1 illustrates hypothetical outcomes, Figure 2B shows the results of a study in which we used the amplitude of the late positive potential (LPP, an ERP component that, as shown in Figure 2A, reflects the motivational relevance of stimuli, irrespective of their hedonic value [27,28]) to measure neuroaffective responses to a wide array of cigarette-related and non-drug related stimuli in 180 smokers interested in quitting [29]. Applying multivariate analyses to the LPP responses (a key step to account for reactivity across all stimulus categories), we identified two neuroaffective reactivity profiles: one is characterized by larger LPP responses to cigarette-related cues than to pleasant images (SCR+) and the other is characterized by larger LPP responses to pleasant images than cigarette-related cues (SCR−). In line with the hypothesis that these profiles capture individual differences in the tendency to attribute motivational relevance to drug-related cues, a trait that should be associated with vulnerability to cue-induced drug seeking behavior, smokers characterized by the SCR+ profile relapsed significantly more often that smokers with the SCR- profile [29]. Since the publication of these findings, we replicated the presence of the two profiles in two studies involving smokers interested in quitting (Figure 2C–D, [30,31]), two studies involving smokers not interested in quitting (Figure 2E, [31], and, not shown in the figure, [32]), one study involving smokers that also abused alcohol [34], and in one study involving individuals with cocaine use disorder (Figure 2F,[35]). Furthermore, in line with animal models indicating that the tendency to attribute high incentive salience to cues predicting rewards might be a trait that also increases vulnerability to maladaptive cue-induced compulsive eating [36,37], we showed that the two profiles are present in nonsmokers when food-related cues instead of drug-related cues are used (Figure 2 G–H, [38,39] and, not shown in the figure [40]). Importantly, in all the studies presented in Figure 2, we validated the potential clinical relevance of the two profiles using behavioral measures rather than self-reports: biologically verified smoking relapse and nicotine self-administration (Figure 2 B–E), saccades directed towards drug-related cues (a behavioral measure of attention, Figure 2 F), eating (Figure 2 G–H). In all but one study in preparation [34], individuals characterized by the SCR+ profile showed higher vulnerability to cue-induced behaviors than individuals characterized by the SCR- profile.
Figure 2: Individual LPP-based neuroaffective profiles are replicable, generalizable, and predictive of vulnerability to maladaptive cue-induced behaviors.
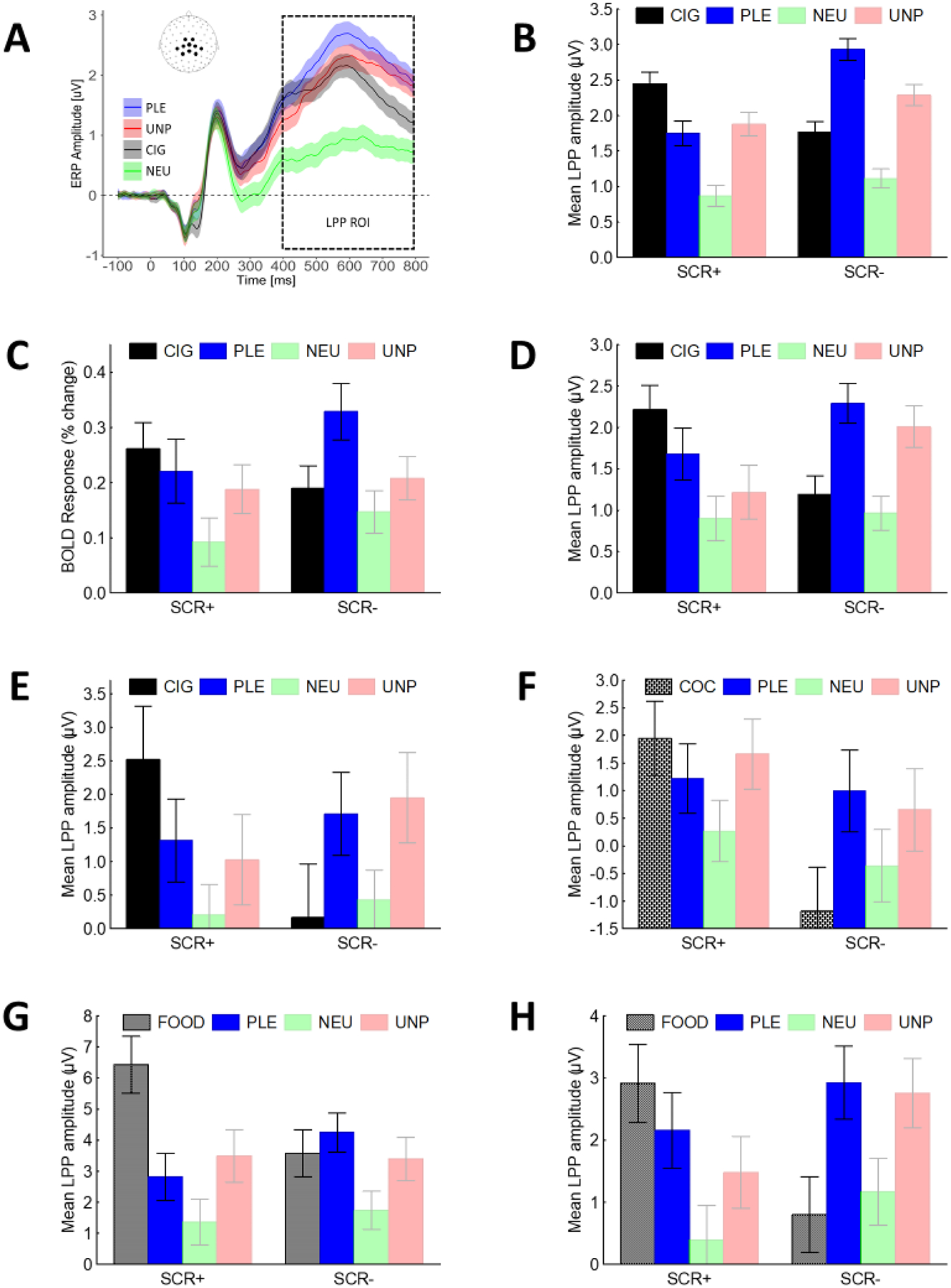
A) During passive picture viewing, motivationally relevant images, including cigarette-related cues, increase the amplitude o fthe late positive potential (LPP) over central and parietal sites; B-H) There are large individual differences in LPP responses to non-drug-related pleasant stimuli and cues predicting rewards (B-E nicotine, F cocaine, G-H food): individuals that react more to cues than to pleasant stimuli (SCR+) are more vulnerable to cue-induced maladaptive behaviors than individuals with the opposite neuroaffective profile (i.e., larger LPP responses to pleasant stimuli than cues predicting rewards, SCR-). Note: LPP=Late Positive Potential, ROI=Region of Interest, CIG=Cigarette-related cues, PLE=Pleasant, NEU=Neutral, UNP=Unpleasant, COC= Cocaine-related cues, FOOD=Food-related cues.
The results outlined above support our first recommendation to always include non-drug-related motivationally relevant pleasant and unpleasant stimuli when trying to determine the extent to which individuals attribute high incentive salience to drug-related cues and to use clinically relevant behaviors (i.e., drug self-administration) rather than self-reports to validate any potential neuromarker identified using this procedure.
2.2. Methodological caveats
The motivational relevance of the non-drug-related images included in the “enhanced” cue reactivity paradigm is likely to influence its outcomes. Pre-clinical studies in monkeys showed that the neural representation of the value assigned to a stimulus adapts to the range of values available in a given situation [41]. Hence, restricting the motivational range of the non-drug-related images included in the enhanced cue reactivity paradigm (e.g., by including only low arousing non-drug related stimuli) might not yield an unbiased and reliable measure of the drug-related stimuli’s incentive salience. It is also important to note that most available standardized picture sets that include non-drug-related motivationally relevant images provide normative values of motivational relevance that are based on self-reports, but previous studies showed that self-reports of motivational relevance do not always correlate with physiological responses [42,43]. To help scientists select the appropriate visual stimuli for studies where neuroaffective responses are the main outcome of interest, we recently published electrophysiological normative responses to emotional, neutral, and cigarette-related images [44]. Relying on these neurophysiological normative responses when selecting the images to include in psychophysiology experiments should increase the rigor and reproducibility of the results obtained with the enhanced cue reactivity paradigm.
2.3. Open questions
Before concluding that the electrophysiological profiles described above can be considered a biomarker of the motivational relevance individuals attribute to cues predicting rewards, several questions must still be answered. For example, while preliminary data indicate that the two profiles are associated with genetic differences [45], these findings are being replicated in a larger sample in an on-going study from our lab. Furthermore, the stability over time of the two profiles and their malleability to pharmacological and other treatments also needs to be established. Finally, as mentioned in Section 2, measuring brain responses to a wide array of motivationally relevant stimuli allows to also assess reactivity to intrinsically pleasant and unpleasant stimuli. This is relevant because reward deficits have also been proposed as a hallmark characteristic of substance use disorders [46]. It is possible that both enhanced reactivity to drug-related cues and blunted reactivity to non-drug-related pleasant stimuli contribute to the SCR+ profile. In fact we initially attributed the SCR+ profile to blunted reactivity to non-drug-related rewards [29,31,45,47]. However, because LPP responses to pleasant stimuli are significantly larger than those evoked by neutral stimuli also in the SCR+, our neurophysiological data do not warrant the conclusion that this profile is associated with reduced reward sensitivity. Furthermore, neither self-reports not behavioral indices of hedonic capacity correlate with the SCR+ profile. We are currently investigating the extent to which the two neuroaffective profiles outlined above map onto other behavioral and physiological indices of hedonic capacity as answering this question is not only theoretically relevant, but also has clinical implications.
3. Recommendation 2: Identifying neuromarkers of the ability to control drug-related decisions, requires measuring brain activity during drug-related decision.
In addition of being vulnerable to cue-induced compulsive drug seeking, individuals with SUDs also have difficulties in controlling drug urges, drug-related decisions, and drug taking [14]. Accordingly, individuals with SUDs show reduced activity in the prefrontal cortical areas that are primarily involved in cognitive control and decision making [17]. However, due to the complexity of the decision-making systems, dysregulated activity in different regions of these networks can generate different deficits and maladaptive behaviors [48] and several tasks have been developed to assess these deficits. In most of these tasks, however, participants do not make drug-related decisions. We thought that identifying neuromarkers of cognitive control during drug related decisions required monitoring brain activity while participants decided whether or not to self-administer nicotine. Drug self-administration procedures have been already used to study human drug-related behaviors [49,50], but usually these paradigms did not include brain imaging recordings. Another advantage of measuring brain activity during a drug self-administration procedure in humans is that the same procedures are commonly used in animal models to study addiction’s neurobehavioral correlates [51], hence adopting it is likely to improve the efficiency of the translational pipeline in addiction research [52,53].
3.1. Example: Phasic EEG theta responses during drug-related decisions predict substance self-administration.
The self-administration procedure that we developed to identify neuromarkers of cognitive control during drug-related decisions is an extension of the enhanced cue reactivity task described above: participants look at a slide show that, per Recommendation 1, includes a wide array of non-drug-related images, and also includes images indicating the impending availability of a reward. In some experiments we used a palatable food option (i.e., candies [38,54]), and in another we used nicotine that was made available to the participant through an electronic nicotine delivery system that could be used during the study [32]. Because EEG is recorded continuously throughout the task, this methodology allows for the collection of brain activity both during the presentation of the cues (e.g., see the results shown in Figure 2 panels E, G, H) and when the actual reward is delivered (see Figure 3 A), to capture when participants, as in real life situations, decide whether to consume the reward or not. To monitor brain activity during the decision-making process, we measured power in the EEG theta frequency band (θ, 4–8 Hz) over midfrontal scalp sites. Previous studies showed that power in the theta band increases over midfrontal sites when participants engage cognitive control mechanisms to inhibit prepotent responses, and monitor and resolve response conflicts during difficult tasks [55–57]. Figure 3 A shows that, in line with this literature, midfrontal theta power increases when a reward becomes available and individuals must decide whether to consume it. Because both rewards and non-reward tokens can be delivered during the task [39], we computed theta power changes for each person under 3 conditions: reward-related decisions, non-reward-related decisions, and passive viewing (i.e., when no decisions are required). Then, using cluster analysis, we grouped participants based on individual theta response patterns: some individuals showed larger theta responses during the reward condition than during the non-reward conditions (θ+), while others had the opposite reactivity profile (θ−, Figure 3 B) [39]. In two studies, we found that these neurocognitive profiles were associated with individual differences in food consumption and (Figure 3 C) nicotine self-administration [58]. While these results should still be considered preliminary, the similarity of the results obtained using two different rewards is in line with theoretical models indicating that substance of abuse hijack brain mechanisms underlying reward responses [59] and suggest that this experimental paradigm should lead to robust and replicable neuromarkers of the ability to control drug-related decisions.
Figure 3: Individual theta-based neurocognitive profiles are associated with vulnerability to nicotine self-administration.
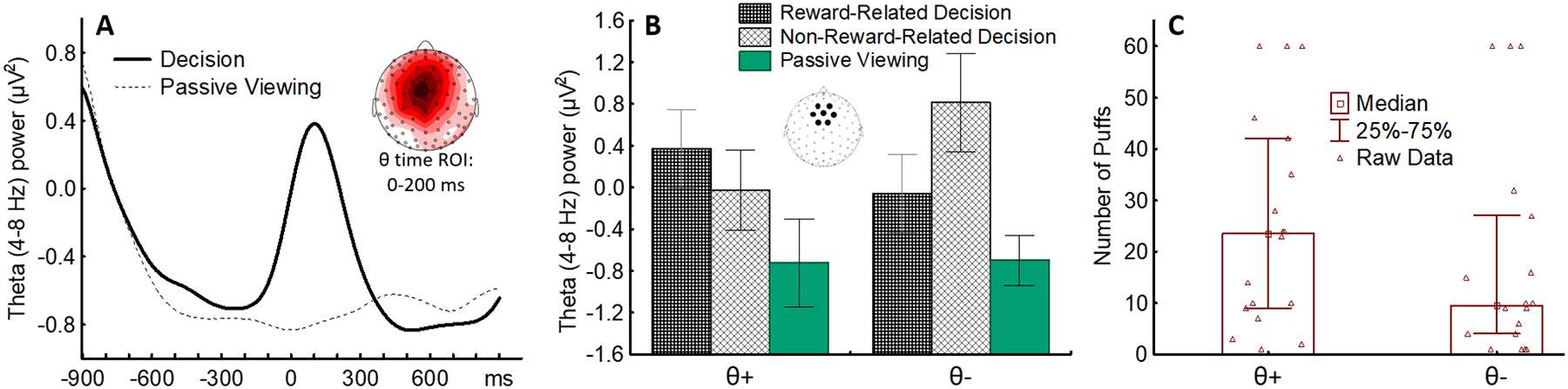
A) Theta power increases over midfrontal sensors when smokers decide whether to self-administer nicotine. B) Cluster analysis yields 2 groups with opposite θ response profiles. C) Smokers showing higher θ responses during nicotine-related decisions (θ+) are more vulnerable to nicotine self-administration than smokers with the opposite neurocognitive profile.
3.2. Open questions
Because we have analyzed theta responses during self-administration procedures only in two experiments, the potential validity of the neurophysiological profiles described above still needs extensive replication. Once its predictive validity and association with individual differences in cognitive control during drug-related decisions are further replicated, it will be possible to determine the extent to which the LPP-based neuroaffective and the theta-based neurocognitive biomarkers correlate. However, our preliminary analyses [39] indicate that information about one biomarker does not provide information about the other, indicating that they are influenced by different affective and cognitive processes. Future studies should be conducted to determine the extent to which this neuromarker is stable over time, is associated with genetic differences, and how treatments designed to improve cognitive control influence it.
4. Conclusions and Final Recommendations.
In summary, we highlighted two methodological issues that have prevented neuroscientists from accurately assessing the neurobiological underpinnings of the tendency to attribute high incentive salience to drug-related cues and the reduced ability to exert cognitive control over drug-related decisions, two main psychological vulnerabilities to SUDs. Our recommendations to overcome these limitations are: 1) Measure the incentive salience attributed to drug-related cues within the motivational context provided by non-drug-related pleasant and unpleasant images varying in motivational relevance; and 2) measure executive control when individuals can immediately self-administer the drug. A third, more general recommendation is to always validate potential neuromarkers against drug-related behaviors (e.g., drug self-administration, biochemically verified long-term abstinence) rather than self-reports of craving [60]. Even though craving is now part of the DSM criteria to diagnose SUDs [61], it can only be self-reported and not objectively assessed. The biases associated with self-report measures are likely to increase uncertainty of outcomes and reduce the predictive value of potential biomarker.
Our data show that following these recommendations, yield replicable neuromarkers that predict vulnerability to nicotine self-administration and smoking relapse during a quit attempt. We showed that these EEG-based neuromarkers can be translated into classifiers deployable in clinical setting to prospectively predict vulnerability to relapse [30] and match patients to treatments [47,62]. Because disorders characterized by poor impulse control share similar psychophysiological mechanisms, we think that the neuroaffective and neurocognitive biomarkers that we identified are likely to be clinically relevant across multiple disorders (e.g., tobacco use disorder, alcohol use disorders, cocaine use disorders, excessive eating). We also think that adapting the cued self-administration procedure described above to the MRI environment will be both scientifically and clinically relevant. The better spatial resolution afforded by fMRI will allow neuroscientists to better understand the interactions between cortical and subcortical networks when individuals are exposed to cues with high incentive salience and make drug-related decisions and could accelerate the development of personalized treatments targets for SUDs.
Acknowledgements
Support for this research was provided by US National Institute of Health (NIH) grants R01DA017073, R01DA024709, and R01DA032581, and by MD Anderson Cancer Center’s Support Grant (P30CA016672) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declarations of Interest: none
References
- [1].Volkow ND, Michaelides M, Baler R, The neuroscience of drug reward and addiction, Physiol. Rev 99 (2019) 2115–2140. 10.1152/physrev.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Robinson TE, Berridge KC, The Neural Basis of Drug Craving: An Incentive-Sensitization Theory of Addiction, Brain Res. Rev 18 (1993) 247–291. [DOI] [PubMed] [Google Scholar]
- [3].Goldstein RZ, Volkow ND, Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications, Nat. Rev. Neurosci 12 (2011) 652–669. 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Berridge KC, From prediction error to incentive salience: Mesolimbic computation of reward motivation, Eur. J. Neurosci 35 (2012) 1124–1143. 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Di Chiara G, Nucleus accumbens shell and core dopamine: Differential role in behavior and addictions, Behav. Brain Res 137 (2002) 75–114. [DOI] [PubMed] [Google Scholar]
- [6].Bardo MT, The Mesolimbic Dopamine Reward System and Drug Addiction, in: Miller PM (Ed.), Biol. Res. Addict, Elsevier Inc., 2013: pp. 209–217. 10.1016/B978-0-12-398335-0.00022-4. [DOI] [Google Scholar]
- [7].Di Chiara G, Bassareo V, Reward system and addiction: what dopamine does and doesn’t do, Curr. Opin. Pharmacol 7 (2007) 69–76. 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [8].Delgado MR, Beer JS, Fellows LK, Huettel SA, Platt ML, Quirk GJ, Schiller D, Viewpoints: Dialogues on the functional role of the ventromedial prefrontal cortex, Nat. Neurosci 19 (2016) 1545–1552. 10.1038/nn.4438. [DOI] [PubMed] [Google Scholar]
- [9].Cofresí RU, Bartholow BD, Piasecki TM, Evidence for incentive salience sensitization as a pathway to alcohol use disorder, Neurosci. Biobehav. Rev 107 (2019) 897–926. 10.1016/j.neubiorev.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hickey C, Peelen MV, Neural mechanisms of incentive salience in naturalistic human vision, Neuron. 85 (2015) 512–518. 10.1016/j.neuron.2014.12.049. [DOI] [PubMed] [Google Scholar]
- [11].Berridge KC, Robinson TE, Liking, wanting, and the incentive-sensitization theory of addiction., Am. Psychol 71 (2016) 670–679. 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Robinson MJF, Robinson TE, Berridge KC, Incentive Salience and the Transition to Addiction, in: Miller PM (Ed.), Biol. Res. Addict, Academic Press, San Diego, CA, 2013: pp. 391–399. 10.1016/B978-0-12-398335-0.00039-X. [DOI] [Google Scholar]
- [13].Volkow ND, Wang GJ, Tomasi D, Baler RD, The addictive dimensionality of obesity, Biol. Psychiatry 73 (2013) 811–818. 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Baler RD, Volkow ND, Drug addiction: the neurobiology of disrupted self-control, Trends Mol. Med 12 (2006) 559–566. 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [15].Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ, Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review, Neuron. 98 (2018) 886–903. 10.1016/j.neuron.2018.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koechlin E, Summerfield C, An information theoretical approach to prefrontal executive function, Trends Cogn. Sci 11 (2007) 229–235. 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [17].McTeague LM, Goodkind MS, Etkin A, Transdiagnostic impairment of cognitive control in mental illness, J. Psychiatr. Res 83 (2016) 37–46. 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luck SJ, Mathalon DH, O’Donnell BF, Hmlinen MS, Spencer KM, Javitt DC, Uhlhaas PJ, A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research, Biol. Psychiatry 70 (2011) 28–34. 10.1016/j.biopsych.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Logothetis NK, What we can do and what we cannot do with fMRI, Nature. 453 (2008) 869–878. 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- [20].Moeller SJ, Paulus MP, Toward biomarkers of the addicted human brain: Using neuroimaging to predict relapse and sustained abstinence in substance use disorder, Prog. Neuro-Psychopharmacology Biol. Psychiatry 80 (2018) 143–154. 10.1016/j.pnpbp.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Houston RJ, Schlienz NJ, Event-Related Potentials as Biomarkers of Behavior Change Mechanisms in Substance Use Disorder Treatment, Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 (2018) 30–40. 10.1016/j.bpsc.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garrison KA, Potenza MN, Neuroimaging and Biomarkers in Addiction Treatment, Curr. Psychiatry Rep 16 (2014). 10.1007/s11920-014-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sutherland MT, Stein EA, Functional Neurocircuits and Neuroimaging Biomarkers of Tobacco Use Disorder, Trends Mol. Med 24 (2018) 129–143. 10.1016/j.molmed.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kapur S, Phillips AG, Insel TR, Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it, Mol. Psychiatry 17 (2012) 1174–1179. 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- [25].Oliver JA, Jentink KKG, Drobes DDJ, Evans DDE, Smokers exhibit biased neural processing of smoking and affective images., Health Psychol. 35 (2016) 866–869. 10.1037/hea0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Versace F, Engelmann JM, Deweese MM, Robinson JD, Green CE, Lam CY, Minnix JA, Karam-Hage M, Wetter DW, Schembre SM, Cinciripini PM, Beyond cue reactivity: Non-drug-related motivationally relevant stimuli are necessary to understand reactivity to drug-related cues., Nicotine Tob. Res 19 (2017) 663–669. 10.1093/ntr/ntx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lang PJ, Bradley MM, Emotion and the motivational brain, Biol. Psychol 84 (2010) 437–450. 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Weinberg A, Hajcak G, Beyond good and evil: the time-course of neural activity elicited by specific picture content., Emotion. 10 (2010) 767–782. 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- [29].Versace F, Lam CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM, Beyond cue reactivity: Blunted brain responses to pleasant stimuli predict long-term smoking abstinence, Addict. Biol 17 (2012) 991–1000. 10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Frank DW, Cinciripini PM, Deweese MM, Karam-Hage MA, Kypriotakis G, Lerman C, Robinson JD, Tyndale RF, Vidrine DJ, Versace F, Toward precision medicine for smoking cessation: Developing a neuroimaging-based classification algorithm to identify smokers at higher risk for relapse, Nicotine Tob. Res 22 (2020) 1277–1284. 10.1093/ntr/ntz211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, Minnix JA, Karam-hage M, Brown VL, Wetter DW, Cinciripini PM, Prequit fMRI responses to pleasant cues and cigarette-related cues predict smoking cessation outcome, Nicotine Tob. Res 16 (2014) 697–708. 10.1093/ntr/ntt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Versace F, Kypriotakis G, Neuroaffective profiles are associated with e-cigarette use, Biorxiv Prepr. (2022). https://www.biorxiv.org/content/10.1101/2022.02.04.479183v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Engelmann JM, Versace F, Gewirtz JC, Cinciripini PM, Individual Differences in Brain Responses to Cigarette-Related Cues and Pleasant Stimuli in Young Smokers, Drug Alcohol Depend. 163 (2016) 229–235. 10.1016/j.drugalcdep.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Robinson JD, Versace F, Cinciripini PM, Lack of evidence that increased substance cue reactivity predicts treatment outcome among smokers who abuse alcohol, In preparation.
- [35].Webber HE, De Dios C, Wardle MC, Suchting R, Green CE, Schmitz JM, Lane SD, Versace F, Electrophysiological Responses to Emotional and Cocaine Cues Reveal Individual Neuroaffective Profiles in Cocaine Users, Exp. Clin. Psychopharmacol (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saunders BT, Robinson TE, Individual variation in resisting temptation: Implications for addiction, Neurosci. Biobehav. Rev 37 (2013) 1955–1975. 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, a Akers C, Clinton SM, Phillips PEM, Akil H, A selective role for dopamine in stimulus-reward learning., Nature. 469 (2011) 53–57. 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Versace F, Frank DW, Stevens EMEM, Deweese MMMM, Guindani M, Schembre SMSM, The reality of “food porn”: Larger brain responses to food-related cues than to erotic images predict cue-induced eating, Psychophysiology. 56 (2019) e13309. 10.1111/psyp.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gibney KD, Kypriotakis G, Versace F, Individual differences in LPP amplitude and theta power predict cue-induced eating, BiorXiv. (2022). 10.1101/2022.03.28.485549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Versace F, Kypriotakis G, Basen-Engquist K, Schembre SM, Heterogeneity in brain reactivity to pleasant and food cues: evidence of sign-tracking in humans., Soc. Cogn. Affect. Neurosci 11 (2016) 604–611. 10.1093/scan/nsv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Padoa-Schioppa C, Range-adapting representation of economic value in the orbitofrontal cortex., J. Neurosci 29 (2009) 14004–14014. 10.1523/JNEUROSCI.3751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bradley MM, Codispoti M, Cuthbert BN, Lang PJ, Emotion and Motivation I: Defensive and Appetitive Reactions in Picture Processing, Emotion. 1 (2001) 276–298. 10.1037/1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- [43].Bradley MM, Sapigao RG, Lang PJ, Sympathetic ANS modulation of pupil diameter in emotional scene perception: Effects of hedonic content, brightness, and contrast, Psychophysiology. 54 (2017) 1419–1435. 10.1111/psyp.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Versace F, Sambuco N, Deweese MM, Cinciripini PM, Electrophysiological normative responses to emotional, neutral, and cigarette-related images, Biorxiv Prepr. (2022). https://www.biorxiv.org/content/10.1101/2022.04.11.487896v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Robinson JD, Versace F, Lam CY, Minnix JA, Engelmann JM, Cui Y, Karam-Hage M, Shete SS, Tomlinson GE, Chen T-L, Wetter DW, Green CE, Cinciripini PM, The CHRNA3 rs578776 variant is associated with an intrinsic reward sensitivity deficit in smokers, Front. Psychiatry 4 (2013) 25–27. 10.3389/fpsyt.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Koob GF, Volkow ND, Neurobiology of addiction: a neurocircuitry analysis, The Lancet Psychiatry. 3 (2016) 760–773. 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cinciripini PM, Green CE, Robinson JD, Karam-Hage MA, Engelmann JM, Minnix JA, Wetter DW, Versace F, Benefits of varenicline vs. bupropion for smoking cessation: A Bayesian Analysis of the interaction of reward sensitivity and treatment, Psychopharmacology (Berl). 234 (2017). 10.1007/s00213-017-4580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Redish AD, Jensen S, Johnson A, A unified framework for addiction: Vulnerabilities in the decision process, Behav. Brain Sci 31 (2008) 415–437. 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Haney M, Self-administration of cocaine, cannabis and heroin in the human laboratory: Benefits and pitfalls, Addict. Biol 14 (2009) 9–21. 10.1111/j.1369-1600.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carter BL, Tiffany ST, The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers, Exp. Clin. Psychopharmacol 9 (2001) 183–190. 10.1037/1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- [51].Sanchis-Segura C, Spanagel R, Behavioural assessment of drug reinforcement and addictive features in rodents: An overview, Addict. Biol 11 (2006) 2–38. 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- [52].Cavanagh JF, Gregg D, Light GA, Olguin SL, Sharp RF, Bismark AW, Bhakta SG, Swerdlow NR, Brigman JL, Young JW, Electrophysiological biomarkers of behavioral dimensions from cross-species paradigms, Transl. Psychiatry 11 (2021). 10.1038/s41398-021-01562-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Moeller SJ, Stoops WW, Cocaine choice procedures in animals, humans, and treatment-seekers: Can we bridge the divide?, Pharmacol. Biochem. Behav 138 (2015) 133–141. 10.1016/j.pbb.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Deweese MM, Claiborne KN, Ng J, Dirba DD, Stewart HL, Schembre SM, Versace F, Dispensing apparatus for use in a cued food delivery task, MethodsX. 2 (2015) 446–457. 10.1016/j.mex.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cavanagh JF, Frank MJ, Frontal theta as a mechanism for cognitive control, Trends Cogn. Sci 18 (2014) 414–421. 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Haciahmet CC, Frings C, Pastötter B, Target Amplification and Distractor Inhibition: Theta Oscillatory Dynamics of Selective Attention in a Flanker Task, Cogn. Affect. Behav. Neurosci 21 (2021) 355–371. 10.3758/s13415-021-00876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nigbur R, Ivanova G, Stürmer B, Theta power as a marker for cognitive interference, Clin. Neurophysiol 122 (2011) 2185–2194. 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- [58].Versace F, Theta-based neurocognitive profiles predict nicotine self-administration, In preparation.
- [59].Volkow ND, Wang GJ, Tomasi D, Baler RD, Obesity and addiction: Neurobiological overlaps, Obes. Rev 14 (2013) 2–18. 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Perkins KA, Does smoking cue-induced craving tell us anything important about nicotine dependence?, Addiction. 104 (2009) 1610–1616. 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- [61].Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF, DSM-5 criteria for substance use disorders: Recommendations and rationale, Am. J. Psychiatry 170 (2013) 834–851. 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cinciripini PM, Green CE, Robinson JD, Minnix JA, Kypriotakis G, Seokhun K, Deweese MM, Karam-Hage MA, Versace F, Personalizing smoking cessation pharmacotherapy using neuroaffective biomarkers., In preparation.


