ABSTRACT
Background: Sepsis-associated encephalopathy (SAE) is a dysfunction of the central nervous system experienced during sepsis with variable clinical and pathophysiologic features. We sought to identify distinct SAE phenotypes in relation to clinical outcomes. Methods: The Medical Information Mart for Intensive Care IV (MIMIC-IV) database and the eICU database were used to conduct a retrospective cohort study. Adult sepsis patients were included and SAE was defined as having a Glasgow Coma Scale (GCS) score ˂15 or delirium. The following our clinical phenotypes were defined as: ischemic-hypoxic, metabolic, mixed (ischemic-hypoxic and metabolic), and unclassified. The primary outcome was in-hospital mortality. Results: The study enrolled 4,120 sepsis patients, 2,239 from MIMIC-IV (including 1,489 patients with SAE, 67%), and 1,881 from eICU (1,291, 69%). For the SAE cohort, 2,780 patients in total were enrolled (median age, 67 years; interquartile range, 56–76.8; 1,589 (57%) were male; median GCS score was 12 [8–14]; median Sequential Organ Failure Assessment score was 6 [4–9]). The SAE phenotype distributions between the MIMIC-IV and eICU cohorts were as follows (39% vs. 35% ischemic-hypoxic, P = 0.043; 38% vs. 40% metabolic, P = 0.239; 15% vs. 15% mixed, P = 0.972; 38% vs. 40% unclassified, P = 0.471). For the overall cohort, the in-hospital mortality for patients with ischemic-hypoxic, metabolic, mixed, or unclassified phenotypes was 33.9% (95% confidence interval, 0.3–0.37), 28.4% (0.26–0.31), 41.5% (0.37–0.46), and 14.2% (0.12–0.16), respectively. In the multivariable logistic analysis, the mixed phenotype was associated with the highest risk of in-hospital mortality after adjusting for age, sex, GCS, and modified Sequential Organ Failure Assessment score (adjusted odds ratio, 2.11; 95% confidence interval, 1.67–2.67; P < 0.001). Conclusions: Four SAE phenotypes had different clinical outcomes. The mixed phenotype had the worst outcomes. Further understanding of these phenotypes in sepsis may improve trial design and targeted SAE management.
KEYWORDS/ABBREVIATIONS: Sepsis-associated encephalopathy, clinical phenotype, large observational database, cohort study, ALT – alanine transaminase, AST – aspartate transaminase, CI – confidence interval, GCS – Glasgow Coma Scale, ICU – intensive care unit, IQR – interquartile range, LOS – length of stay, MIMIC-IV – Medical Information Mart for Intensive Care IV, OR – odds ratio, RRT – renal replacement therapy, SAE – sepsis-associated encephalopathy, SD – standard deviation, SOFA – Sequential Organ Failure Assessment, Spo2 – pulse oximetry
INTRODUCTION
Sepsis is life-threatening multiple organ failure caused by a dysregulated host response to infection (1). Sepsis-associated encephalopathy (SAE) is the dysfunction of the central nervous system during sepsis, which includes variable clinical features from delirium to coma (2,3). According to broad diagnostic criteria, the incidence of SAE reporting varies fairly from 9% to 76% (2–4). Its pathophysiology comprises neuroinflammation (5,6), cerebral ischemia (7), and metabolic disturbances (8,9). Given the heterogeneity of conditions involved, specific therapeutic targets for brain dysfunction in sepsis remain limited. Identifying clinical phenotypes in sepsis presenting with similar biological and prognostic features might lead to more personalized therapy and focused clinical trial design.
Recent studies in the critically ill with heterogeneous diseases have highlighted subphenotypes based on underlying pathophysiology, such as in sepsis and delirium (10–12). Xu et al. (10) identified four clinically- defined and Sequential Organ Failure Assessment (SOFA) trajectory-based sepsis subphenotypes, which provided further understanding of host-pathogen responses. A large prospective cohort study showed several delirium phenotypes predicted different severities of long-term cognitive impairment (11). The hypoxic and sepsis-associated delirium phenotypes predicted worse long-term cognition while patients with a metabolic phenotype did not show such an association. A recent retrospective study suggested that as among other factors, acute renal failure, hypoglycemia, or hypernatremia may play a role in the pathophysiology of SAE (13). However, whether the differential patterns of organ dysfunction accompanied with SAE are associated with distinct clinical phenotypes remains unexplored.
In this study based on electronic health databases, we intended to identify sepsis phenotypes of similar pathophysiologic features and clinical outcomes, their prevalence, and related clinical outcomes.
METHODS
Data source
For this retrospective cohort study, cohorts were extracted from two large, publicly available databases: the Medical Information Mart for Intensive Care IV (MIMIC-IV) IV database (14,15) and the eICU Collaborative Research Database (16,17). The MIMIC-IV database holds the records for more than 380,000 hospitalized patients admitted to intensive care units (ICUs) of the Beth Israel Deaconess Medical Centre in Boston, Massachusetts, between 2008 and 2019. The eICU database contained high-granularity data of more than 200,000 admissions from 335 ICUs at 208 hospitals located throughout the United States between 2014 and 2015.
One author acquired access to the two data sets. The study was exempt from our institutional review board approval because the databases used deidentified data and also carried preexisting institutional review board approval. The study was reported in accordance with the Reporting of Studies Conducted Using Observational Routinely Collected Health Data statement (18) and the Strengthening the Reporting of Observational Studies in Epidemiology statement (19).
Study population
We included patients with (1) sepsis as the cause of ICU admission, (2) age of 18 years or older and younger than 89 years, (3) ICU stay duration of at least 24 hours, (4) we considered only the first ICU stay for patients with multiple ICU admissions. We identified sepsis patients within 24 hours after ICU admission. Sepsis was defined based on Sepsis-3 criteria, a widely used method for identifying sepsis from 2016 (20). The onset of sepsis was defined when a patient had SOFA ≥2 and suspicion of infection. We extracted variables used in SOFA and screened infection, respectively. The calculation window of the SOFA is 24 hours. The suspected infection was defined as (1) antibiotic taken after the specimen was culture, but no more than 72 hours, (2) or culture followed by an antibiotic in subsequent 24 hours. The time of suspected infection: (1) the culture time (if before antibiotic) or (2) the antibiotic time (if before culture). Patients with an acute brain injury (e.g., meningitis, encephalitis, status epilepticus, traumatic brain injury, or stroke) were excluded. The detailed criteria and codes are shown in supplementary materials 1–8, http://links.lww.com/SHK/B637. Sepsis-associated encephalopathy was defined within 24 hours after ICU admission as a GCS score less than 15 or abnormal neurological findings consistent with delirium (13). Patients with unavailable GCS scores were excluded. For each patient, we used the lowest score on the GCS available 24 hours after starting an ICU admission.
Clinical variables
We retrospectively collected from the MIMIC-IV and eICU databases: (1) age and gender; (2) vital signs, including: systolic blood pressure, diastolic blood pressure, MAP, heart rate, respiratory rate, pulse oximetry (Spo2), and body core temperature; (3) laboratory tests, including: blood gases, complete blood cell counts, liver, renal, and coagulation test results; (4) infection-related parameters, including: site of infection and bacteremia; (5) severity of illness assessed using SOFA score, and a modified SOFA score excluding the neurological component (13). The worst values were calculated within 24 hours from ICU admission. The primary outcome was in-hospital mortality. Secondary outcomes included ICU and hospital lengths of stay (LOS), the use of mechanical ventilation, or renal replacement therapy (RRT).
Clinical phenotypes
Adapted from both a previous study (11) and hypothesized mechanisms, the following four clinical SAE phenotypes were defined: ischemic-hypoxic, metabolic, mixed (ischemic-hypoxic and metabolic), and unclassified phenotype. The detailed definitions were as follows:
• Ischemic-hypoxic SAE
Hypoxemia or septic shock
• Metabolic SAE
Serum urea nitrogen > 17.85 mmol/L or
Glucose < 2.5 mmol/L or
INR > 2.5 and (aspartate transaminase [AST] or alanine transaminase [ALT]) > 200 U/L or
Sodium < 120 mmol/L or
Sodium > 160 mmol/L
• Mixed SAE
Qualifies under both ischemic-hypoxic and metabolic phenotypes
• Unclassified SAE
None of the above
Hypoxemia was identified as two or more 15-min intervals during which the lowest Spo2 was less than 90%. Septic shock was defined by the Sepsis-3 criteria, which combined any vasopressor initiation, lactate level >2 mmol/L, and MAP <65 mm Hg (1).
Statistical analysis
Continuous variables were presented as median with interquartile range (IQR) or mean with standard deviation (SD) and compared between groups by Wilcoxon nonparametric tests or Student t tests as appropriate. Categorical variables were expressed as frequencies with percentages (%) and analyzed using χ2 tests.
Bonferroni-adjusted post hoc tests were used for multiple comparison.
A logistic regression model was built to test the association between SAE phenotype and categorical outcomes. The multivariate analyses were adjusted on age, sex, GCS, and modified SOFA score. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported in the logistic regression analyses.
To determine replicability of the clinical phenotypes, the same analyses were conducted in the MIMIC-IV and eICU databases. We statistically evaluated the reproducibility and consistency as follows: (1) comparing the prevalence and in-hospital mortality of each phenotype between the two cohorts and (2) visualization of the phenotype prevalence and in-hospital mortality.
Missing values in all data were less than 12% (Fig. S1, http://links.lww.com/SHK/B638). Missing values were imputed by multiple imputation with the chained equations method (21). We performed a sensitivity analysis to compare original cases versus the complete imputed data set. We used the R programming language (version 4.1.3) for all statistical analyses. All tests were two-tailed, and P values less than 0.05 were considered to be statistically significant.
RESULTS
Cohort characteristics
The overall cohort enrolled 4,120 sepsis patients, 2,239 from MIMIC-IV (of which 1,489 had SAE, 67%), and 1,881 from eICU (1,291 had SAE, 69%) (Fig. 1). Detailed baseline characteristics of the sepsis cohorts are shown in Supplemental Tables S1 and S2, http://links.lww.com/SHK/B638. For clinical phenotypes, the SAE cohort had more ischemic-hypoxic (37% vs. 30%, P < 0.001), metabolic (39% vs. 28%, P < 0.001), and mixed phenotype (15% vs. 9%, P < 0.001) than non-SAE cohort (Table S3, Fig. S2, http://links.lww.com/SHK/B638). Of the 2,780 patients with SAE, 57% were male and had median age of 67 years (56–76.8) (Table 1). The median GCS score was 12 (8–14) and the median SOFA score was 6 (4–9). The overall in-hospital mortality was 23% (95% CI, 0.21–0.25). Patients in the eICU cohort had a lower baseline GCS score (10 [8–13] vs. 13 [10–14]) and a higher modified SOFA score (6 [4–8] vs. 4 [2–6]) than the MIMIC-IV cohort. As for outcomes, the patients from the MIMIC-IV cohort required more mechanical ventilation (74% vs. 42%) and renal replacement therapy (22% vs. 12%) than SAE patients from the eICU database. The median ICU LOS in the MIMIC-IV and eICU cohorts was 4.1 days (2.2–8.9) and 3.7 days (2–7.2), respectively.
Fig. 1.

Workflow of study. GCS Glasgow Coma Scale, SAE, sepsis-associated encephalopathy.
Table 1.
Baseline characteristics of SAE patients from the MIMIC-IV and eICU databases
| Variables | Total (N = 2,780) | MIMIC-IV (n = 1,489) |
eICU (n = 1,291) |
|---|---|---|---|
| Age, median (IQR) | 67 (56–76.8) | 67 (55.9–76.6) | 67 (56–77) |
| Sex, male (%) | 1,589 (57) | 899 (60) | 690 (53) |
| GCS, median (IQR) | 12 (8–14) | 13 (10–14) | 10 (8–13) |
| Delirium, n (%) | 605 (22) | 560 (38) | 45 (3) |
| Phenotype, n (%) | |||
| Ischemic-hypoxic | 1,034 (37) | 580 (39) | 454 (35) |
| Metabolic | 1,084 (39) | 565 (38) | 519 (40) |
| Mixed | 426 (15) | 229 (15) | 197 (15) |
| Unclassified | 1,088 (39) | 573 (38) | 515 (40) |
| Hypoxemia | 196 (7) | 72 (5) | 124 (10) |
| Septic shock | 946 (34) | 553 (37) | 393 (30) |
| SOFA score, median (IQR) | 6 (4–9) | 4 (3–6) | 8 (6–10) |
| Modified SOFA score, median (IQR) | 5 (3–7) | 4 (2–6) | 6 (4–8) |
| Vital signs, median (IQR) | |||
| Systolic blood pressure, mm Hg | 83 (73–92) | 83 (74–90) | 83 (73–95) |
| Diastolic blood pressure, mm Hg | 43 (36–50) | 42 (36–48) | 44 (36–52) |
| Mean arterial pressure, mm Hg | 54 (47–62) | 54 (46–60) | 55 (47–64) |
| Heart rate, min−1 | 113 (97–130) | 112 (95–129) | 114 (98–130) |
| Respiratory rate, min−1 | 30 (25–35) | 30 (25–34) | 30 (25–36) |
| Spo2, % | 91 (88–94) | 91 (88–94) | 92 (87–95) |
| Temperature, °C | 37.4 (37–38.1) | 37.3 (37–38) | 37.5 (37–38.2) |
| Biological parameters, median (IQR) | |||
| pH | 7.3 (7.2–7.4) | 7.3 (7.2–7.4) | 7.3 (7.2–7.4) |
| Po2, mm Hg | 62 (41–85) | 54 (38–81) | 69 (54.3–89.1) |
| Pco2, mm Hg | 42 (36–50) | 44 (37–50) | 41 (34–50.4) |
| Lactate, mmol/L | 2.4 (1.5–4.3) | 2.3 (1.4–4) | 2.6 (1.6–4.7) |
| WBC, ×109/L | 16.1 (10.6–23.2) | 15.7 (10.1–22.7) | 16.9 (11.2–23.6) |
| Hemoglobin, g/L | 94 (79–110) | 93 (79–109) | 94 (80–112) |
| Hematocrit, % | 28.7 (24.3–33.2) | 28.4 (24.1–32.6) | 28.9 (24.5–33.9) |
| Platelets, ×109/L | 155 (94–234) | 149 (90–233) | 161 (99–235) |
| Creatinine, umol/L | 153.8 (88.4–277.8) | 150.3 (88.4–282.9) | 156.5 (90.2–274) |
| BUN, mmol/L | 13.9 (8.2–21.8) | 13.9 (7.5–21.8) | 13.9 (9–21.4) |
| Minimum glucose, mmol/L | 6.1 (4.8–7.7) | 6.3 (4.9–8.1) | 5.8 (4.8–7.3) |
| Maximum glucose, mmol/L | 9.1 (6.9–12.2) | 8.9 (6.7–12.1) | 9.2 (7.2–12.6) |
| Minimum sodium, mmol/L | 136 (132–139) | 136 (133–139) | 136 (131–139) |
| Maximum sodium, mmol/L | 140 (136–143) | 139 (136–142) | 140 (137–144) |
| ALT, U/L | 33 (19–79) | 38 (20–93) | 30 (18–69) |
| AST, U/L | 52 (28–124.2) | 61 (32–142) | 43 (24–104) |
| Bilirubin, umol/L | 17.1 (8.6–41) | 20.5 (8.6–58.1) | 15.4 (8.6–27.4) |
| PT (s) | 16.6 (14.3–22) | 16.8 (14.5–22.7) | 16.4 (14.2–21.6) |
| INR | 1.5 (1.2–2) | 1.5 (1.3–2.1) | 1.4 (1.2–1.9) |
| Infection source, n (%) | |||
| Pulmonary infection | 1,272 (46) | 703 (47) | 569 (44) |
| Urinary tract infection | 848 (31) | 514 (35) | 334 (26) |
| Intra-abdominal infection | 564 (20) | 363 (24) | 201 (16) |
| Skin soft tissue infection | 292 (11) | 154 (10) | 138 (11) |
| Bacteremia | 376 (14) | 314 (21) | 62 (5) |
| Outcomes comparison | |||
| Mechanical ventilation, n (%) | 1,652 (59) | 1,105 (74) | 547 (42) |
| Renal replacement therapy, n (%) | 477 (17) | 323 (22) | 154 (12) |
| ICU LOS, median (IQR), d | 3.8 (2.2–8) | 4.1 (2.2–8.9) | 3.7 (2–7.2) |
| Hospital LOS, median (IQR), d | 11 (6–20) | 13.2 (7.3–24.6) | 9 (5–15) |
| In-hospital mortality, n (%) | 637 (23) | 370 (25) | 267 (21) |
Comparisons across SAE clinical phenotype
The prevalence of SAE phenotypes according to cohort is shown in Table 1. The overall cohort had 1,034 (37%), 1,084 (39%), 426 (15%), and 1,088 (39%) patients in the clinical phenotypes of ischemic-hypoxic, metabolic, mixed, and unclassified, respectively. The SAE phenotype distributions by cohort from the MIMIC-IV and eICU databases were as follows (ischemic-hypoxic: 39% vs. 35%, P = 0.043; metabolic: 38% vs. 40%, P = 0.239; mixed: 15% vs. 15%, P = 0.972; unclassified: 38% vs. 40%, P = 0.471). Table 2 shows patient characteristics among the clinical SAE phenotypes. In general, patients in the ischemic-hypoxic and metabolic phenotypes were more critically ill than those in unclassified phenotype. The mixed phenotype was associated with a worse GCS score (median 10 [6–13] vs. 13 [9–14]), higher SOFA score (median 9 [6–12] vs. 5 [3–7]) and more severe biological parameters than the unclassified phenotype (Table 2). Among all infection sources, pulmonary infections occurred in 1272 of SAE patients (46%). For outcome comparisons among the phenotypes, the median ICU LOS was 4.9 (2.8–10.1) days in those with the ischemic-hypoxic phenotype, 3.9 (2.2–7.5) days in the metabolic phenotype, 5 (2.6–9.2) days in the mixed phenotype, and 3 (1.9–6) days in the unclassified phenotype. Patient characteristics and outcomes comparison among the clinical phenotypes of SAE after adjusting the significance level by the Bonferroni correction are shown in Supplemental Tables S4, http://links.lww.com/SHK/B638.
Table 2.
Patient characteristics and outcomes comparison among the clinical phenotypes of SAE
| Variables | Total (N = 2,780) | Ischemic-hypoxic* (n = 608) | Metabolic* (n = 658) | Mixed (n = 426) | Unclassified (n = 1,088) | P |
|---|---|---|---|---|---|---|
| Database, n (%) | 0.1 | |||||
| MIMIC | 1,489 (54) | 351 (58) | 336 (51) | 229 (54) | 573 (53) | |
| eICU | 1,291 (46) | 257 (42) | 322 (49) | 197 (46) | 515 (47) | |
| Age, median (IQR) | 67 (56–76.8) | 68 (56.4–76) | 68 (57.3–78) | 67.4 (58.4–76.2) | 65 (54–76) | <0.001 |
| Sex, male (%) | 1,589 (57) | 299 (49) | 411 (62) | 267 (63) | 612 (56) | <0.001 |
| GCS, median (IQR) | 12 (8–14) | 11 (7–14) | 12 (8–14) | 10 (6–13) | 13 (9–14) | <0.001 |
| Delirium, n (%) | 605 (22) | 147 (24) | 138 (21) | 96 (23) | 224 (21) | 0.34 |
| SOFA score, median (IQR) | 6 (4–9) | 6 (4–9) | 7 (4–9) | 9 (6–12) | 5 (3–7) | <0.001 |
| Modified SOFA score, median (IQR) | 5 (3–7) | 5 (3–7.2) | 5 (3–7) | 7 (5–9.8) | 4 (2–5) | <0.001 |
| Vital signs, median (IQR) | ||||||
| Systolic blood pressure, mm Hg | 83 (73–92) | 77 (68–83) | 87 (78–97) | 74 (63–83) | 87 (80–98) | <0.001 |
| Diastolic blood pressure, mm Hg | 43 (36–50) | 39 (33–45) | 43 (36–50) | 38 (29–44) | 47 (39–54) | <0.001 |
| Mean arterial pressure, mm Hg | 54 (47–62) | 50 (43–56) | 56 (50–64) | 49 (37–55) | 59 (51–67) | <0.001 |
| Heart rate, min−1 | 113 (97–130) | 121 (105–137) | 105 (90–121) | 118 (99–136) | 112 (97–127) | <0.001 |
| Respiratory rate, min−1 | 30 (25–35) | 32 (27–37) | 28 (24–33) | 30 (26–35) | 29 (24–35) | <0.001 |
| Spo2, % | 91 (88–94) | 89 (83–93) | 92 (90–95) | 90 (84–93) | 92 (90–94) | <0.001 |
| Temperature, °C | 37.4 (37–38.1) | 37.6 (37.2–38.4) | 37.2 (36.8–37.7) | 37.3 (36.9–38) | 37.5 (37–38.2) | <0.001 |
| Biological parameters, median (IQR) | ||||||
| pH | 7.3 (7.2–7.4) | 7.3 (7.2–7.4) | 7.3 (7.3–7.4) | 7.2 (7.1–7.3) | 7.4 (7.3–7.4) | <0.001 |
| Po2, mm Hg | 62 (41–85) | 58.2 (40–81) | 67 (43–90.9) | 57 (38–75.8) | 66 (44–86) | <0.001 |
| Pco2, mm Hg | 42 (36–50) | 44 (37–53) | 40 (33–48) | 43.3 (36–53) | 42.9 (36–50) | <0.001 |
| Lactate, mmol/L | 2.4 (1.5–4.3) | 3.8 (2.6–6.1) | 1.8 (1.3–2.6) | 4.5 (2.8–8.7) | 1.8 (1.3–2.9) | <0.001 |
| WBC, ×109/L | 16.1 (10.6–23.2) | 16.8 (10.3–24.9) | 15.6 (10.3–22.3) | 21 (13.9–29.3) | 15 (10–20.6) | <0.001 |
| Hemoglobin, g/L | 94 (79–110) | 96 (82–114) | 89.5 (75–106) | 91 (76–109) | 95 (82–112) | <0.001 |
| Hematocrit, % | 28.7 (24.3–33.2) | 29.2 (24.8–34.2) | 27.6 (23.1–32.4) | 28.4 (23–33.3) | 29.1 (25.2–33.3) | <0.001 |
| Platelets, ×109/L | 155 (94–234) | 146 (87–223.2) | 152 (96–230.8) | 130 (70–204) | 171 (107.8–256) | <0.001 |
| Creatinine, umol/L | 153.8 (88.4–277.8) | 131.3 (88.4–193.6) | 265.2 (162.7–395.4) | 282.9 (194.5–415.5) | 97.2 (70.7–150.3) | <0.001 |
| BUN, mmol/L | 13.9 (8.2–21.8) | 10.5 (7.1–13.5) | 25.1 (20.2–32.2) | 23.6 (19.9–31.1) | 9.4 (6–13.1) | <0.001 |
| Minimum glucose–mmol/L | 6.1 (4.8–7.7) | 6.3 (5–8.1) | 5.8 (4.7–7.4) | 6 (4.4–7.9) | 6.1 (5.1–7.7) | <0.001 |
| Maximum glucose, mmol/L | 9.1 (6.9–12.2) | 9.4 (7.4–12.6) | 8.8 (6.8–12.8) | 10.6 (7.7–15.4) | 8.5 (6.7–10.8) | <0.001 |
| Minimum sodium, mmol/L | 136 (132–139) | 136 (133–139) | 136 (131–140) | 135 (130.2–138) | 137 (133–139) | <0.001 |
| Maximum sodium, mmol/L | 140 (136–143) | 140 (136–142) | 140 (136–144) | 139 (136–143) | 139 (137–142) | 0.212 |
| ALT, U/L | 33 (19–79) | 35 (20–92.2) | 33 (19–74) | 60 (23–267) | 28 (17–56.2) | <0.001 |
| AST, U/L | 52 (28–124.2) | 61 (31–156) | 53 (28–110) | 103 (38.2–442.2) | 42 (22–84) | <0.001 |
| Bilirubin, umol/L | 17.1 (8.6–41) | 20.5 (11.5–51.3) | 15.4 (8.6–35.5) | 27.4 (13.7–61.1) | 13.7 (8.6–29.1) | <0.001 |
| PT, s | 16.6 (14.3–22) | 17.5 (14.9–22.6) | 16.3 (13.9–23.5) | 19.6 (15.8–30) | 15.8 (13.8–19.6) | <0.001 |
| INR | 1.5 (1.2–2) | 1.6 (1.3–2.1) | 1.4 (1.2–2.2) | 1.8 (1.4–2.9) | 1.4 (1.2–1.8) | <0.001 |
| Infection source, n (%) | ||||||
| Pulmonary infection | 1,272 (46) | 293 (48) | 244 (37) | 203 (48) | 532 (49) | <0.001 |
| Urinary tract infection | 848 (31) | 169 (28) | 238 (36) | 135 (32) | 306 (28) | 0.002 |
| Intra-abdominal infection | 564 (20) | 139 (23) | 134 (20) | 81 (19) | 210 (19) | 0.311 |
| Skin soft tissue infection | 292 (11) | 72 (12) | 69 (10) | 56 (13) | 95 (9) | 0.047 |
| Bacteremia | 376 (14) | 109 (18) | 88 (13) | 73 (17) | 106 (10) | <0.001 |
| Outcomes comparison | ||||||
| Mechanical ventilation, n (%) | 1,652 (59) | 449 (74) | 311 (47) | 319 (75) | 573 (53) | <0.001 |
| Renal replacement therapy, n (%) | 477 (17) | 99 (16) | 139 (21) | 148 (35) | 91 (8) | <0.001 |
| ICU LOS, median (IQR), d | 3.8 (2.2–8) | 4.9 (2.8–10.1) | 3.9 (2.2–7.5) | 5 (2.6–9.2) | 3 (1.9–6) | <0.001 |
| Hospital LOS, median (IQR), d | 11 (6–20) | 11.2 (6–22.7) | 10 (6–21) | 11.7 (5–19) | 10.1 (6–17) | 0.022 |
| In-hospital mortality, n (%) | 637 (23) | 174 (29) | 131 (20) | 177 (42) | 155 (14) | <0.001 |
*The ischemic-hypoxic and metabolic phenotypes excluded patients from the mixed phenotype.
Sepsis-associated encephalopathy clinical phenotype and outcomes
For the overall cohort, the in-hospital mortality of patients with the ischemic-hypoxic, metabolic, mixed, and unclassified phenotypes was 33.9% (95% CI, 0.30–0.37), 28.4% (95% CI, 0.26–0.31), 41.5% (95% CI, 0.37–0.46), and 14.2% (95% CI, 0.12–0.16), respectively. There was no significant difference in in-hospital mortality for every clinical phenotype between the two cohorts (Fig. 2). The associations between SAE phenotypes and in-hospital mortality are displayed in Figure 3 and Figure S3, http://links.lww.com/SHK/B638. In the overall cohort, the mixed phenotype (including ischemic-hypoxic and metabolic) was associated with a higher risk of in-hospital mortality (OR, 2.93; 95% CI, 2.35–3.64; P < 0.001). After adjusting for age, sex, GCS, and modified SOFA score, the association still remained (adjusted OR, 2.11; 95% CI, 1.67–2.67; P < 0.001). The ischemic-hypoxic phenotype predicted more use of mechanical ventilation (OR, 2.82; 95% CI, 2.38–3.33; P < 0.001; adjusted OR, 2.86; 95% CI, 2.39–3.42; P < 0.001; Table S5a, http://links.lww.com/SHK/B638) and the metabolic phenotype required more renal replacement therapy (OR, 2.85; 95% CI, 2.33–3.49; P < 0.001; adjusted OR, 2.37; 95% CI, 1.91–2.93; P < 0.001; Table S5b, http://links.lww.com/SHK/B638).
Fig. 2.

In-hospital mortality of sepsis-associated encephalopathy phenotypes according to the cohort.
Fig. 3.
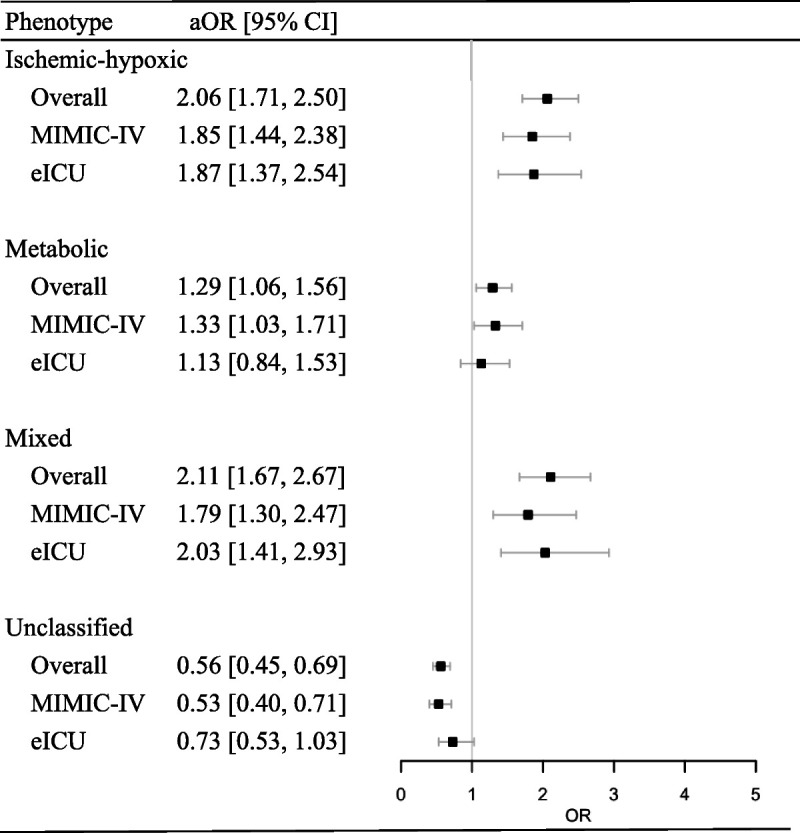
Associations between sepsis-associated encephalopathy phenotypes and in-hospital mortality with multivariable logistic regression analysis. aOR adjusted odds ratio, CI confidence interval.
DISCUSSION
In this retrospective analysis of large electronic health data set analysis, more than half of the sepsis patients had encephalopathy. Based on previous studies (11) and hypothesized mechanisms including ischemic-hypoxic, metabolic, mixed, and unclassified phenotypes, metabolic and unclassified were the most common phenotypes. The mixed phenotype was associated with higher risk of in-hospital mortality and longer hospital stay.
The current Sepsis-3 definition for sepsis emphasizes life-threatening organ dysfunction and acute brain dysfunction as common features (1,22). Various factors contribute to the development of SAE, including neuroinflammation, renal and liver dysfunction, and neurovascular changes, and drugs (23,24). Our study highlighted the multiple organ failures that can accompany SAE, and categorized these into four phenotypes using clinical variables captured from patients’ electronic health records. The prevalence and relation to clinical outcomes of each SAE phenotype were consistent across the two health data sets. These clinical phenotypes may inform the design of future study.
Consistent with our study, previous clinical studies reported on the likely mechanism contributing to SAE. A study including 30 patients with severe sepsis or septic shock suggested that dysfunction of the cerebrovascular autoregulation system was associated with sepsis-associated delirium (4). A prospective study conducted by Zhang et al. (25) also showed that the percentage of patients in shock was higher in the SAE group than in the non-SAE group. The ischemic-hypoxic phenotype may be more likely to develop SAE when impaired cerebrovascular autoregulation in sepsis leads to cerebral hypoperfusion. A recent case series showed that bedside autoregulation monitoring can identify blood pressure ranges associated with autoregulation and preserved cerebral perfusion in SAE (26). Metabolic disturbances may be one of the hallmarks of the pathophysiology of SAE (27–29). A study conducted by Zhu et al. (27) identified a correlation between 4-hydroxyphenylacetic acid and disorders of consciousness. A retrospective analysis of a multicenter database demonstrated some common metabolic disturbances and acute renal failure as potentially modifiable factors contributing to SAE (13). Another study from our center found that nonhepatic hyperammonemia occurred more often in patients with SAE (30), and this may be a potential therapeutic target for SAE (31).
The mixed phenotype (combined ischemic-hypoxic and metabolic dysfunction) may represent severe organ failure during sepsis or septic shock. In this condition, brain homeostasis deteriorated, experiencing permanent brain injury, even death (32).
The mixed phenotype was the smallest group in both databases, and the mortality may be biased by the group size, which should be taken into consideration when interpreting the findings. Although we have adjusted the covariates, there could be residual confounding due to unmeasured covariates. The unclassified phenotype accounted for 39% of patients in the SAE cohort, which indicates that patients without clear organ failure besides encephalopathy were well represented in our study. This unclassified phenotype had more mild alterations in mental status during sepsis, which may predict a more reversible brain dysfunction. The brain is vulnerable to being affected by a variety of diseases outside of the central nervous system and the severity of encephalopathy may be distinct (33,34).
Our study had several limitations that should be taken into consideration when interpreting our results. First, the definition of SAE was according to GCS score or delirium assessment, which may be confounded by many factors (e.g., prior sedative agents, mechanical ventilation). Although many clinical studies have adopted similar criteria, the subjectivity of this element should not be ignored (35). Second, we focused on the SAE phenotype at ICU admission, and we did not identify the dynamic development of the phenotypes. Third, the absence of follow-up data in the two databases did not allow for assessment of the long-term outcomes for these patients. Fourth, the unclassified group is quite large and the variables in our definition may not fully represent the characteristics of the phenotype. However, there are no specific neuroinflammation markers in the databases, which could be verified in the future prospective study. Despite these limitations, our study identified clinical phenotypes of SAE patients using real-world data related to clinical practice. Moreover, we used distinct data sets for identification and validation, which should enhance reproducibility and generalizability.
CONCLUSIONS
We reported four SAE phenotypes with different clinical outcomes, of which the mixed phenotype was associated with the worst outcomes. Further understanding of differential phenotypes in sepsis may inform the design of future clinical trials.
Supplementary Material
Footnotes
This work was supported by the National High Level Hospital Clinical Research Funding (serial number 2022-PUMCH-B-109) and CAMS Innovation Fund for Medical Sciences (CIFMS) (serial number 2021-I2M-1-020).
The authors report no conflict of interests.
Consent obtained for use of MIMIC-IV and eICU databases.
The data sets supporting the conclusions of this article are included within the article and its additional files.
XL and YL set up the idea for this study. MQ, YG, SY, and ZG collected the data. XL, CG, and HZ analyzed the data. XL wrote the first draft of the manuscript. JHW, DA, and YL revised the final version of the manuscript. All authors read and approved the final manuscript.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.shockjournal.com).
Contributor Information
Xin Lu, Email: luxin61@126.com.
Mubing Qin, Email: qmbb719@163.com.
Joseph Harold Walline, Email: jwallinemd@gmail.com.
Yanxia Gao, Email: gaoyanxiazzu@163.com.
Shiyuan Yu, Email: 362384870@qq.com.
Zengzheng Ge, Email: gezengzheng@126.com.
Chao Gong, Email: gongchao20210513@163.com.
Huadong Zhu, Email: zhuhuadong1970@126.com.
Djillali Annane, Email: djillali.annane@aphp.fr.
REFERENCES
- 1.Singer M Deutschman CS Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eidelman LA Putterman D Putterman C, et al. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA. 1996;275(6):470–473. [PubMed] [Google Scholar]
- 3.Wu L Ai ML Feng Q, et al. Serum glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 for diagnosis of sepsis-associated encephalopathy and outcome prognostication. J Crit Care. 2019;52:172–179. [DOI] [PubMed] [Google Scholar]
- 4.Schramm P Klein KU Falkenberg L, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. 2012;16(5):R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourhy L Mazeraud A Bozza FA, et al. Neuro-inflammatory response and brain-peripheral crosstalk in sepsis and stroke. Front Immunol. 2022;13:834649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017;17(1):49–59. [DOI] [PubMed] [Google Scholar]
- 7.Orhun G Tüzün E Bilgiç B, et al. Brain volume changes in patients with acute brain dysfunction due to sepsis. Neurocrit Care. 2020;32(2):459–468. [DOI] [PubMed] [Google Scholar]
- 8.Mazeraud A Righy C Bouchereau E, et al. Septic-associated encephalopathy: a comprehensive review. Neurotherapeutics. 2020;17(2):392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan X Yang K Xiao Q, et al. Central role of microglia in sepsis-associated encephalopathy: from mechanism to therapy. Front Immunol. 2022;13:929316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z Mao C Su C, et al. Sepsis subphenotyping based on organ dysfunction trajectory. Crit Care. 2022;26(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard TD Thompson JL Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters-Sengers H Butler JM Uhel F, et al. MARS consortium . Source-specific host response and outcomes in critically ill patients with sepsis: a prospective cohort study. Intensive Care Med. 2022;48(1):92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaga S Shime N Sonneville R, et al. Risk factors for sepsis-associated encephalopathy. Intensive Care Med. 2017;43(10):1548–1549. [DOI] [PubMed] [Google Scholar]
- 14.Johnson A Bulgarelli L Pollard T, et al. MIMIC-IV (version 1.0). PhysioNet. 2021. doi: 10.13026/s6n6-xd98. [DOI] [Google Scholar]
- 15.Goldberger AL Amaral LA Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–E220. [DOI] [PubMed] [Google Scholar]
- 16.Pollard TJ Johnson AEW Raffa JD, et al. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018;5:180178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard T Johnson A Raffa J, et al. eICU Collaborative Research Database (version 2.0). PhysioNet. 2019. doi: 10.13026/C2WM1R. [DOI] [Google Scholar]
- 18.Benchimol EI Smeeth L Guttmann A, et al. , RECORD Working Committee . The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E Altman DG Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 20.Shankar-Hari M Phillips GS Levy ML, et al. , Sepsis Definitions Task Force . Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buuren S, Groothuis-Oudshoorn KGM. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 22.Ehler J Petzold A Sharshar T, et al. Biomarker panel to differentiate brain injury from brain dysfunction in patients with sepsis-associated encephalopathy. Crit Care Med. 2020;48(5):e436–e437. [DOI] [PubMed] [Google Scholar]
- 23.Manabe T, Heneka MT. Cerebral dysfunctions caused by sepsis during ageing. Nat Rev Immunol. 2022;22(7):444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu SY Ge ZZ Xiang J, et al. Is rosuvastatin protective against sepsis-associated encephalopathy? A secondary analysis of the SAILS trial. World J Emerg Med. 2022;13(5):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang LN Wang XH Wu L, et al. Diagnostic and predictive levels of calcium-binding protein A8 and tumor necrosis factor receptor-associated factor 6 in sepsis-associated encephalopathy: a prospective observational study. Chin Med J (Engl). 2016;129(14):1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenblatt K Walker KA Goodson C, et al. Cerebral autoregulation-guided optimal blood pressure in Sepsis-associated encephalopathy: a case series. J Intensive Care Med. 2020;35(12):1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J Zhang M Han T, et al. Exploring the biomarkers of sepsis-associated encephalopathy (SAE): metabolomics evidence from gas chromatography-mass spectrometry. Biomed Res Int. 2019;2019:2612849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi T Uchino H Elmér E, et al. Disease outcome and brain metabolomics of cyclophilin-D knockout mice in sepsis. Int J Mol Sci. 2022;23(2):961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Browne CA Clarke G Fitzgerald P, et al. Distinct post-sepsis induced neurochemical alterations in two mouse strains. Brain Behav Immun. 2022;104:39–53. [DOI] [PubMed] [Google Scholar]
- 30.Zhao L Walline JH Gao Y, et al. Prognostic role of ammonia in critical care patients without known hepatic disease. Front Med (Lausanne). 2020;7:589825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L Li Y Wang Y, et al. Non-hepatic hyperammonemia: a potential therapeutic target for sepsis-associated encephalopathy. CNS Neurol Disord Drug Targets. 2022;21(9):738–751. [DOI] [PubMed] [Google Scholar]
- 32.Ehler J Barrett LK Taylor V, et al. Translational evidence for two distinct patterns of neuroaxonal injury in sepsis: a longitudinal, prospective translational study. Crit Care. 2017;21(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins RO. Acquired brain injury following Sepsis. Crit Care Med. 2019;47(11):1658–1659. [DOI] [PubMed] [Google Scholar]
- 34.Ehlenbach WJ Sonnen JA Montine TJ, et al. Association between sepsis and microvascular brain injury. Crit Care Med. 2019;47(11):1531–1538. [DOI] [PubMed] [Google Scholar]
- 35.Iacobone E Bailly-Salin J Polito A, et al. Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med. 2009;37(10 Suppl):S331–S336. [DOI] [PubMed] [Google Scholar]


