Objective:
To report our experience with the combination of radical surgical excision and intestinal transplantation in patients with recurrent pseudomyxoma peritonei (PMP) not amenable to further cytoreductive surgery (CRS).
Background:
CRS and heated intraoperative peritoneal chemotherapy are effective treatments for many patients with PMP. In patients with extensive small bowel involvement or nonresectable recurrence, disease progression results in small bowel obstruction, nutritional failure, and fistulation, with resulting abdominal wall failure.
Methods:
Between 2013 and 2022, patients with PMP who had a nutritional failure and were not suitable for further CRS underwent radical debulking and intestinal transplantation at our centre.
Results:
Fifteen patients underwent radical exenteration of affected intra-abdominal organs and transplantation adapted according to the individual case. Eight patients had isolated small bowel transplantation and 7 patients underwent modified multivisceral transplantation. In addition, in 7 patients with significant abdominal wall tumor involvement, a full-thickness vascularized abdominal wall transplant was performed. Two of the 15 patients died within 90 days due to surgically related complications. Actuarial 1-year and 5-year patient survivals were 79% and 55%, respectively. The majority of the patients had significant improvement in quality of life after transplantation. Progression/recurrence of disease was detected in 91% of patients followed up for more than 6 months.
Conclusion:
Intestinal/multivisceral transplantation enables a more radical approach to the management of PMP than can be achieved with conventional surgical methods and is suitable for patients for whom there is no conventional surgical option. This complex surgical intervention requires the combined skills of both peritoneal malignancy and transplant teams.
Key Words: abdominal wall transplant, pseudomyxoma peritonei, small bowel transplant
Pseudomyxoma peritonei (PMP) is an uncommon clinical entity characterized by mucinous ascites and mucinous tumor implants in the peritoneal cavity. In most cases, PMP progresses slowly but relentlessly, rarely developing lymphatic or distant metastases. The majority arise from a perforated mucinous appendix tumor, though PMP can arise from other intra-abdominal organs such as the ovary, urachus, colon, or pancreas. The majority are histologically low grade, although 20% may show high-grade cellular atypia and some signet ring cell morphology.1,2 The standard of care is complete cytoreductive surgery to remove all macroscopic tumors with visceral resections and peritonectomies combined with hyperthermic intraperitoneal chemotherapy (HIPEC), usually, Mitomycin C administered intraoperatively, after tumor resection.3
However, despite complete cytoreductive surgery and HIPEC, the disease recurs in 22% to 44% of patients who may or may not, be suitable for further cytoreductive surgery.4 In some patients, complete tumor removal is not achievable, almost always due to extensive small bowel involvement, and maximal tumor debulking is the best that can be achieved by conventional means, leading to the inevitable progression of residual disease.4,5 When no further surgical options exist, typically due to extensive small bowel involvement, systemic anticancer treatment may be considered to control the disease, though it has not been proven to be effective. Commonly intestinal fistulation and failure will necessitate parenteral nutrition (PN). As patients develop progressive abdominal distention with nutritional and abdominal wall failure, effective palliation becomes difficult or impossible: the terminal phase of this disease is often very distressing. (Figs. 1A–C).
FIGURE 1.
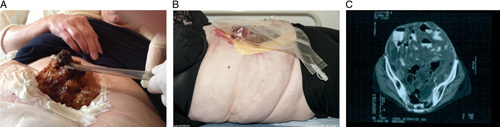
A, Pseudomyxoma peritonei eroding through a midline incision (patient at home and relative suctioning necrotic areas of mucin). B, Expert stoma care to manage fistulating disease (note eosin paint to protect skin and femoral line for home parenteral nutrition). C, Pseudomyxoma with extensive inoperable small bowel involvement “Scrabbled egg” appearance on computed tomography scan.
Intestinal transplantation is now a proven and established therapeutic option in an increasing range of conditions but is associated with a higher complication rate and lower survival than other solid organ transplants such as kidney or liver transplants. The indications for intestinal transplantation are varied,6 and include slow-growing malignancies that are nonresectable due to extensive involvement of vital viscera such as desmoid and neuroendocrine tumors.
In this paper, we present the first series of patients in whom intestinal transplantation was undertaken where conventional surgical options were not possible due to extensive involvement of the small bowel. For patients with significant abdominal wall involvement where the abdomen could not be closed after transplantation, an abdominal wall transplant was carried out. The application of transplantation to PMP was initially driven by a patient whose bravery and perseverance brought together 2 UK national specialist services from different hospitals (peritoneal malignancy in Basingstoke and intestinal transplantation in Oxford) in pursuit of a novel solution to an intractable problem, that of end-stage pseudomyxoma7 (Fig. 2).
FIGURE 2.

Steve Prescott on the cover page of his autobiography—an inspiring patient who initiated this work.
METHODS
Permission to undertake this pilot study was obtained from the Oxford University Hospitals NHS Trust Governance committee and the UK National Adult Small Intestinal Transplant forum.
Study Design
A cohort study of 15 consecutive intestinal and modified multivisceral transplants performed for PMP in a single centre in the UK.
Patient Selection and Preoperative Workup
Criteria for consideration were: (1) Extensive PMP with a peritoneal cancer index of 30 to 39, where no conventional operative solution remained due to involvement of small bowel. (2) Low-grade disease as per the PSOGI pathologic classification2 or high-grade disease with slow time to progression (1 patient with high-grade signet ring cell pathology 7 years from original surgery). (3) Nutritional failure either requiring or about to commence, PN. These selection criteria were based on our own observational data that life expectancy for PMP patients requiring PN is between 6 and 12 months.8
A computed tomography (CT) scan of the neck, thorax, abdomen, and pelvis was undertaken to exclude distant metastasis and also to delineate the venous and arterial anatomy. Only patients considered physically and psychologically fit for surgery were accepted for transplant. Physical fitness for surgery was evaluated by an attending anesthetist using a combination of a 6-minute walk test, cardiopulmonary exercise testing, 2D echo, and pulmonary function tests. Patients were deemed psychologically fit after a formal evaluation by a transplant psychologist/psychiatrist. Patient’s perception of treatment and its benefits, motivation, anxiety, depression, family, and social support were considered in the evaluation. All transplants were ABO blood group and HLA compatible, but no other tissue matching was considered mandatory. Donors and recipients were, however, matched for cytomegalovirus status (ie, avoidance of transplanting cytomegalovirus (CMV)-positive organs into CMV-negative patients), due to recognition of the adverse outcomes of CMV disease in patients undergoing intestinal transplantation.
Surgical Technique
Donor Operation
A modified multivisceral (MMV) graft incorporating the stomach, pancreaticoduodenal complex, small bowel, and the right colon was retrieved from all the donors as described by Cruz et al.9 In addition, a full-thickness abdominal wall graft based on the inferior epigastric vessels was retrieved as previously described by our group.10
Explant
The recipient operation was started as soon as it was clear that the donor organs were suitable for transplantation, to maximize the time available for what was often a prolonged explant procedure. The involved bowel was mobilized and explanted: in half the patients the extent of involvement with the tumor necessitated the removal of the duodenum, pancreas, and stomach as well as small and large intestine (Fig. 3A). The choice of the graft was determined by the extent of the involvement of the abdominal viscera, with the aim of transplanting the minimum amount of donor tissue that still allowed a clearance of the tumor. In some patients, there was very little recurrence in the upper abdomen and it was possible to preserve the stomach, duodenum, and pancreas. Although efforts were made to try and achieve complete clearance of the tumor, in some cases small amounts of residual disease had to be left for reasons of safety, for example, around the porta hepatis or diaphragm. In some cases, a high-power diathermy liver capsulectomy was performed to remove disease from the surface of the liver.
FIGURE 3.
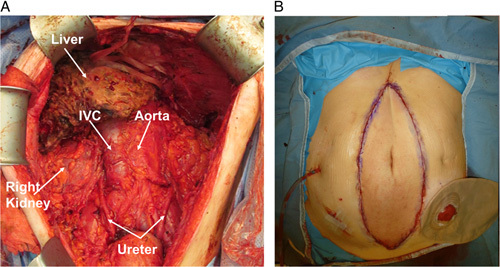
A, Complete cytoreduction achieved after explant. B, Abdominal wall transplant used when there was a loss of abdominal domain. Intra-abdominal drain on the right side of the abdomen and ileostomy covered by a stoma bag on the left side of the abdomen.
Transplant
The donor graft was tailored to the specific needs of the recipient and either an MMV or isolated intestinal graft was prepared. In most cases, the right side of the colon was included in the transplant block. Vascular and enteric reconstructions were carried out according to the needs of the particular case. For isolated intestinal grafts, vascular reconstruction was performed between the donor and native superior mesenteric vessels. For MMV grafts, a short length of donor thoracic aorta was used as a conduit from the infra-renal aorta and anastomosed to a Carrel patch of donor superior mesenteric artery and celiac artery and the venous outflow was by anastomosis to the recipient portal vein.
Abdominal Wall Reconstruction
The abdominal wall was closed using a range of techniques: in some cases direct closure was possible; in others, a full-thickness abdominal wall graft was inserted using a technique previously described (see Fig. 3B)10; in some cases donor abdominal fascia was used (nonvascularized) with primary closure of the skin.
Immunosuppression
Induction Immunosuppression
Patients received 500 mg methylprednisolone intravenously before reperfusion. Intravenous alemtuzumab 30 mg, was started intraoperatively after reperfusion, with a second dose 24 hours later.
Maintenance Immunosuppression
Tacrolimus maintenance therapy was used (target trough level 10–12 ng/mL in the first 6 months and 8–10 ng/mL afterward). All patients since 2018, have had the addition of either azathioprine (50–75 mg daily) or mycophenolate mofetil (250–500 mg twice daily) and/or prednisolone (5–10 mg daily).
Rejection Diagnosis and Treatment
Rejection of the small intestine was diagnosed with endoscopy and biopsy and graded using criteria developed at the VIII International small bowel transplant symposium.11,12 In patients with abdominal wall grafts, skin biopsies were undertaken if there was a rash and graded using the 2007 Banff criteria.13 Treatment of rejection of skin or intestine consisted of 3 daily doses of methylprednisolone intravenous and adjustment of immunosuppressive agents. If rejection persisted or intensified, then additional treatment with antithymocyte globulin was instituted.
Definitions
Residual Disease
Any tumor left behind at the time of the explant either unintentionally or intentionally, if tumor masses were close to prominent structures.
Recurrent Disease
Any new tumor deposits noted on abdominal CT scan performed after 3 months (allowing time for perioperative changes to resolve).
Progressive Disease
Any increase in the size of the residual disease.
Death-censored Intestinal Graft Failure
This was defined as any of the following events occurring after an intestinal transplant:
Graft enterectomy for any cause [anastomotic leak, bleeding, ischemia, rejection, infection, malignancy, or graft-versus-host disease (GVHD)].
Relisting for an intestinal graft transplant.
Recommenced on total parenteral nutrition (TPN) or inability to come off TPN after 3 months.
Health-related quality of life (QOL) was measured pre and postoperatively using the EQ-5D-5L instrument, used with permission.14 This instrument comprises of descriptive system questionnaire and visual analog score (EQ VAS). The descriptive system comprises of 5 dimensions, each describing a different aspect of health, such as mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. In this study, these health states have been reported individually and also converted to EQ index value, a single summary value, which reflects the health of the individual in comparison to the general population. EQ VAS elicits an individual’s rating of their overall current health.
Statistical Analysis
All continuous variables were reported as mean with SD or median with range and all categorical variables were reported as number (n) and percentage (%). Normality of continuous data was tested using the Shapiro-Wilk test. Continuous data were compared using either paired t test or Wilcoxon signed-rank test, depending on their distribution. These tests were used to compare the magnitude and direction of difference between paired ordinal variables. Kaplan-Meier survival analysis was used to analyze patient survival and death-censored graft survival data. This study was exempt from institutional review board approval as it was a retrospective observational study looking at service development.
RESULTS
Demographics
In total, 15 patients underwent intestinal transplantation for PMP (Supplemental Digital Content Table 1, http://links.lww.com/SLA/E359), which illustrates the baseline characteristics, patient, and graft outcomes). The median age at transplant was 47.3 (range, 36–58) years. The duration from initial diagnosis of PMP to transplant ranged from 2 to 12 years. The median duration of TPN before the transplant was 3 (0–18) months, 12 out of 15 patients were on TPN and the other 3 patients were in nutritional decline and approaching TPN.
One further patient with multiple enterocutaneous (EC) fistula with intestinal obstruction and on TPN underwent surgery with the intent to undertake transplantation. However, at surgery, it proved possible to mobilize 200 cm of proximal jejunum and avoid the need for transplantation. This patient was weaned off PN and is currently well after 3 years albeit with some progression of residual disease.
Operative Data
The intraoperative observations are summarized in Table 1. Eight patients (53%) received isolated intestinal transplants and the rest received modified multivisceral transplant grafts. The 6 patients with a peritoneal cancer index <39 (due to less upper abdominal disease) all had isolated small bowel transplants. Primary skin closure was achieved in 8/15 patients, including abdominal wall fascia in 3 cases, and full-thickness abdominal wall transplantation was performed in 7/15 patients. The median red blood cell transfusion requirement was 7 (1–35) units and the median duration of surgery was 13 (8–18.3) hours. The transfusion requirement and duration of surgery reduced progressively over time. The median duration of ITU and hospital stay of index admission were 3 (range, 1–49) days and 37 (0–137) days, respectively.
TABLE 1.
EQ-5D-5L Health-related QOL Instrument
| Cohort with paired pre and postintestinal transplant data | |||||
|---|---|---|---|---|---|
| Preoperative mean (N=11) | SD | Postoperative mean (N=11) | SD | P | |
| Mobility | 2 | 1.2 | 1.6 | 1 | 0.54 |
| Self-care | 1.6 | 0.8 | 1.3 | 0.6 | 0.17 |
| Usual activity (n=10) | 3.4 | 1.1 | 2 | 1.1 | 0.03 |
| Pain/discomfort | 3.5 | 1.5 | 1.7 | 0.8 | 0.01 |
| Anxiety/depression | 2.2 | 1.2 | 1.5 | 1 | 0.25 |
| EQ index value | 0.532 | 0.3 | 0.831 | 0.2 | 0.03 |
| EQ VAS | 37.81 | 27.8 | 71.5 | 20.9 | 0.01 |
Statistically significant p values appear in bold text
Patient Survival
At a median follow-up of 4.59 years (range, 0.18–8.68), there have been 6 deaths: 1 patient died <1-month posttransplant; 2 patients died (13%) between 1 and 6 months; 3 patients (20%) died beyond 6 months. One-year patient survival was 79% (95% CI, 48%-93%) and the actuarial 5-year survival was 55% (95% CI, 25%-77%). The causes of mortality were as follows: anastomotic leak (24 days posttransplant); upper gastrointestinal bleed (69 days posttransplant); GVHD (181 days posttransplant); multifactorial–EC fistula, PN dependence, renal failure, and poor QOL with patient opting for palliative care (1001 days posttransplant), progression of disease (1204 days posttransplant); post-transplant lymphoproliferative disorder (at 1300 days). The longest survivor to date is now over 7 years from surgery (Supplemental Digital Content Table 1, http://links.lww.com/SLA/E359, which illustrates the baseline characteristics, patient and graft outcomes).
Graft Survival
One-year death-censored graft survival was 77% (95% CI, 43%-92%) and the actuarial 5-year death-censored graft survival was 56% (95% CI, 23%-80%).
Rejection
A total of 10 episodes of acute cellular rejection (ACR) episodes were seen in 7/15 (47%) of intestinal allografts. Eight episodes of ACR were seen within the first year of the transplant. A total of 5 episodes of ACR were seen on skin biopsies in 3/12 (25%) of patients with either a vascularized composite allograft or sentinel skin flap. All five of these episodes were seen within the first year of the transplant.
Enterocutaneous fistulas developed in 3 patients (20%). Ureteric stricture/obstruction occurred in 3 patients (20%). Two patients (13%) had vascular complications. An entero-vesical fistula developed in 1 patient (6.6%) with progressive PMP, renal failure needing hemodialysis was seen in 1 patient (6.6%), GVHD developed in 1 (6.6%), and posttransplant lymphoproliferative disorder in 1 (6.6%) (Supplemental Digital Content Table 1, http://links.lww.com/SLA/E359, which illustrates the baseline characteristics, patient and graft outcomes).
Graft Function
Twelve of the 14 patients who survived more than a month were completely weaned off TPN—2 patients developed an EC fistula soon after transplantation and have never come off PN. Freedom from home PN (HPN) at 1 year was achieved in 72% (8/11) patients. However, in the longer term, two of these 8 patients needed supplemental HPN–one due to posttransplant lymphoproliferative disorder requiring intensive chemotherapy and a second patient due to recurrent disease in the pelvis.
Progression or Recurrence of Tumor
This was seen in 91% of patients with at least 6 months of survival/follow-up. In this group, the median time to progression/recurrence (as identified on CT scanning) after transplantation was 363 days (range, 110–727).
Quality of Life
The EQ-5D-5L health-related QOL instrument data show significant improvements were seen in some aspects, including usual activity (mean score, 3.4 vs 2; P value=0.03) and pain/discomfort (mean score, 3.5 vs 1.7; P=0.01). However, this instrument also suggests that other aspects of health including mobility, self-care, and anxiety/depression did not significantly improve after intestinal transplantation. The data also show a significant increase in EQ index value (mean score, 0.532 vs 0.831; P value=0.03) and EQ VAS score (mean score, 37.8 vs 71.5; P value=0.01), which suggest improvement in overall health (Table 1).
DISCUSSION
This study shows that MMV and small bowel transplantation for end-stage PMP is feasible, in selected patients, for whom there is no option for further conventional surgery. The results of this, the first series of patients treated in this way, show 1-year and 5-year survivals of 78% and 55%, respectively with a significant improvement in aspects of QOL in the majority of patients.
One of the key aspects was selecting patients who were young with low-grade disease, fit enough to undergo this extensive surgery as well as ensuring there were no conventional operative approaches possible. Unexpectedly, and on occasions despite very high-volume disease, it was possible to achieve a near complete cytoreduction, especially in patients who had had a previously open and close laparotomy (Fig. 3A). As the program evolved, we felt it was appropriate that patients be listed for transplant earlier–that is, when intestinal failure was imminent and when they were being considered for HPN to try and ensure optimal fitness for surgery pending listing and availability of a transplant donor.
We have seen progression, or recurrent, disease in 91% of patients with at least 6-month survival and follow-up after transplantation. One patient developed lung and bone metastases, which is unusual in PMP, but these developed over a prolonged period several years from surgery. Despite the recurrence, only 1/15 patients died due to recurrent/progressive disease at 3½ years to date. Encouraged by this experience, we have now transplanted 1 patient with defined high-grade signet cell disease pathologically, albeit their disease was progressing at a very slow rate, with currently a good outcome. Moreover, the results are likely to improve with increasing experience.
All the patients had abdominal wall involvement at the transplant. The key factor preventing successful primary abdominal wall closure in some patients was a significant loss of abdominal domain in 7/15 patients.15–17 The tumor and associated EC fistulae infiltrate and destroy abdominal wall tissue, which is not available for closure causing loss of domain. After the excision of a large portion of the abdominal wall, there may be insufficient tissue to achieve primary closure. The risk to transplanted organs of an over-tight abdominal closure is well-known and best avoided. Careful multidisciplinary evaluation of the abdominal wall should be a part of the pretransplant evaluation process. With increasing experience, it was possible to achieve primary closure in an increasing proportion of patients. Even in patients with extensive abdominal wall involvement, there may be sufficient redundancy of abdominal wall transplant due to massive abdominal distension to avoid abdominal wall transplant.
The 1-year survival of 79% is comparable to international standards for patients undergoing intestinal transplantation. Although the 5-year survival of 55% is somewhat lower than the 60% to 70% survival reported for non-PMP intestinal transplants, nonetheless this is much greater than the average survival of 6 to 12 months in patients with nonresectable PMP managed on PN8; indeed, it is also better than 5-year survival for other major cancer surgery such as pancreatic cancer surgery.8,18
Transplantation is already a widely-accepted therapy for selected patients with hepatocellular carcinoma with good long-term outcomes.19 Liver and intestinal transplants are already considered for slow-growing malignancies such as hepatic epitheloid hemangioendothelioma, neuroendocrine tumors, and desmoid tumors.20,21 However, transplantation for adenocarcinomas has been uncommon with limited experience due to justified concerns of early recurrences and optimal organ utilization due to organ shortage. There is emerging evidence of the benefits in liver transplantation for cholangiocarcinoma or for nonresectable colorectal cancer liver metastases, though high recurrence rates are again being noted. Despite reasonable concerns regarding high PMP recurrence rates, the hope is that results will improve with better patient selection and increasing experience. The current experience with transplantation for PMP adds further evidence of the benefit of transplantation in selected patients with adenocarcinomas. Our experience suggests that despite a high recurrence in patients on immunosuppression, the recurrent disease is indolent in the majority of the patients; indeed to date, only one of 15 patient’s death is attributable to recurrent disease.
The issue of organ utilization is key to the application of transplantation for patients with malignant disease: in liver transplantation, the demand for donor organs is so intense that, in many countries, listing for transplantation is limited to patients with a 5-year posttransplant survival prognosis of >50%. This excludes many patients with hepatocellular carcinoma and is at the centre of the ongoing current debate about liver transplantation for secondary colorectal cancer. However, the situation is different for patients with PMP who do not require a liver transplant: there is limited demand for intestinal transplantation and organ availability is therefore not a limiting factor.
This study has several limitations including the small sample size and the highly selected patient population. However, we are encouraged by this preliminary experience, although there is no well-matched comparator group.
In conclusion, we have shown that it is possible to perform intestinal transplantation in selected patients and to achieve long-term survival and improvement in QOL. There are ongoing questions concerning the timing of surgery, its role in higher-grade disease, and whether the addition of HIPEC at the explantation of the tumor might improve disease control. We hope to explore these further as we expand the program.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Srikanth Reddy, Email: srikanth.reddy@ouh.nhs.uk.
Sai Rithin Punjala, Email: punjalarithin@gmail.com.
Philip Allan, Email: Philip.Allan@ouh.nhs.uk.
Anil Vaidya, Email: anil.vaidya@nds.ox.ac.uk.
Deeplaxmi P. Borle, Email: deeplaxmiborle@gmail.com.
Venkatesha Udupa, Email: Venkatesha.Udupa@ouh.nhs.uk.
Alison Smith, Email: Alison.Smith@ouh.nhs.uk.
Lisa Vokes, Email: Lisa.Vokes@ouh.nhs.uk.
Georgios Vrakas, Email: georgios.vrakas@surgery.ufl.edu.
Faheez Mohamed, Email: Faheez.Mohamed@hhft.nhs.uk.
Sanjeev Dayal, Email: Sanjeev.Dayal@hhft.nhs.uk.
Brendan Moran, Email: Brendan.Moran@hhft.nhs.uk.
Peter J. Friend, Email: peter.friend@nds.ox.ac.uk.
Tom Cecil, Email: Tom.Cecil@nhs.net.
REFERENCES
- 1.Carr NJ, Finch J, Ilesley IC, et al. Pathology and prognosis in pseudomyxoma peritonei: a review of 274 cases. J Clin Pathol. 2012;65:919–923. [DOI] [PubMed] [Google Scholar]
- 2.Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi process. Am J Surg Pathol. 2016;40:14–26. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadi N, Kostadinov D, Sakata S, et al. Managing recurrent pseudomyxoma peritonei in 430 patients after complete cytoreduction and HIPEC: a dilemma for patients and surgeons. Ann Surg Oncol. 2021;28:7809–7820. [DOI] [PubMed] [Google Scholar]
- 5.Lord AC, Shihab O, Chandrakumaran K, et al. Recurrence and outcome after complete tumour removal and hyperthermic intraperitoneal chemotherapy in 512 patients with pseudomyxoma peritonei from perforated appendiceal mucinous tumours. Eur J Surg Oncol. 2015;41:396–399. [DOI] [PubMed] [Google Scholar]
- 6.Loo L, Vrakas G, Reddy S, et al. Intestinal transplantation: a review. Curr Opin Gastroenterol. 2017;33:203–211. [DOI] [PubMed] [Google Scholar]
- 7.Steve Prescott MC, Prescott L. One in a Million: My Story: Vertical Editions; 2014. [Google Scholar]
- 8.Swain D, Mason G, Yates A, et al. Outcomes of home parenteral nutrition in 34 patients with intestinal failure from recurrent or progressive peritoneal malignancy of gastro-intestinal tract origin. Eur J Clin Nutr. 2021;75:856–858. [DOI] [PubMed] [Google Scholar]
- 9.Cruz RJ, Jr, Costa G, Bond G, et al. Modified “liver-sparing” multivisceral transplant with preserved native spleen, pancreas, and duodenum: technique and long-term outcome. J Gastrointest Surg. 2010;14:1709–1721. [DOI] [PubMed] [Google Scholar]
- 10.Giele H, Vaidya A, Reddy S, et al. Current state of abdominal wall transplantation. Curr Opin Organ Transplant. 2016;21:159–164. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz P, Bagni A, Brown R, et al. Histological criteria for the identification of acute cellular rejection in human small bowel allografts: results of the pathology workshop at the VIII International Small Bowel Transplant Symposium. Transplant Proc. 2004;36:335–337. [DOI] [PubMed] [Google Scholar]
- 12.Phillip Ruiz JG, Tekin A, Nishida S, et al. Kirk AD, Knechtle SJ, Larsen CP, Madsen JC, Pearson TC, Webber SA. Histological syndromes of intestinal allograft rejection and recurrent disease. Textbook of Organ Transplantation, 1st edn. John Wiley & Sons, Ltd; 2014;Chapter 86:1011–1022. [Google Scholar]
- 13.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8:1396–1400. [DOI] [PubMed] [Google Scholar]
- 14.Janssen MF, Bonsel GJ, Luo N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. Pharmacoeconomics. 2018;36:675–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexandrides IJ, Liu P, Marshall DM, et al. Abdominal wall closure after intestinal transplantation. Plast Reconstr Surg. 2000;106:805–812. [DOI] [PubMed] [Google Scholar]
- 16.Nery JR, Weppler D, DeFaria W, et al. Is the graft too big or too small? Technical variations to overcome size incongruity in visceral organ transplantation. Transplant Proc. 1998;30:2640–2641. [DOI] [PubMed] [Google Scholar]
- 17.Carlsen BT, Farmer DG, Busuttil RW, et al. Incidence and management of abdominal wall defects after intestinal and multivisceral transplantation. Plast Reconstr Surg. 2007;119:1247–1255. [DOI] [PubMed] [Google Scholar]
- 18.Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10:16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203–217. [DOI] [PubMed] [Google Scholar]
- 20.Clift AK, Frilling A. Liver transplantation and multivisceral transplantation in the management of patients with advanced neuroendocrine tumours. World J Gastroenterol. 2018;24:2152–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatzipetrou MA, Tzakis AG, Pinna AD, et al. Intestinal transplantation for the treatment of desmoid tumors associated with familial adenomatous polyposis. Surgery. 2001;129:277–281. [DOI] [PubMed] [Google Scholar]


