Figure 3. CT disassembly in the ER.
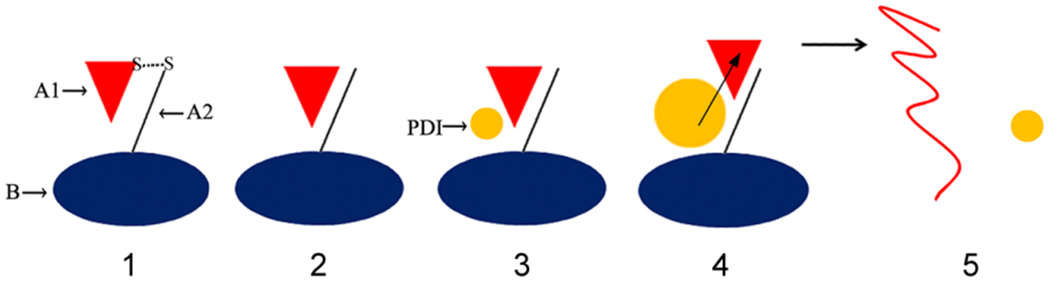
(1) CT travels from the cell surface to the ER as an intact, disulfide-linked holotoxin. (2) The CTA1/CTA2 disulfide bond is reduced in the ER [97, 98], but CTA1 remains associated with CTA2/CTB5 [50, 100–102] through extensive non-covalent contacts. (3) Reduced PDI binds to holotoxin-associated CTA1 [50, 102]. (4) PDI partially unfolds upon contact with CTA1, and the expanded hydrodynamic size of unfolded PDI acts as a wedge to dislodge CTA1 from CTA2/CTB5 [103]. (5) The dissociated CTA1 subunit unfolds spontaneously at 37°C [52], which consequently displaces its PDI binding partner [50]. PDI regains its native conformation after release from CTA1 [103], while disordered CTA1 is treated as a substrate for ERAD-mediated translocation to the cytosol [49, 51].
