FIG 3.
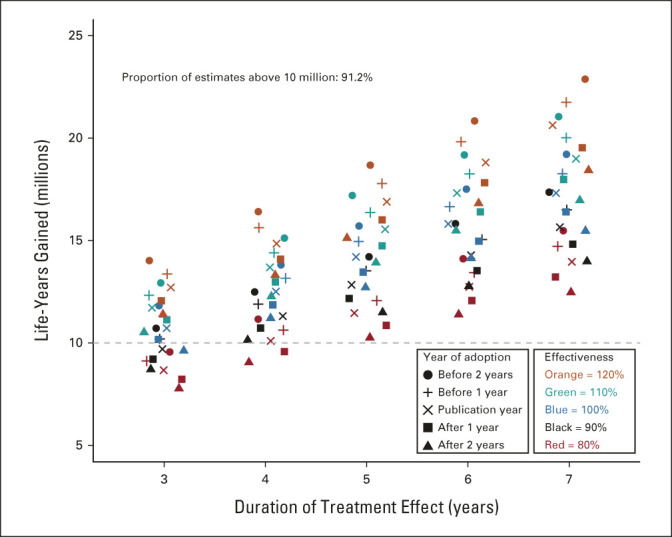
Life-years gained by treatment duration, effectiveness, and year of adoption. Although the base-case model assumed that the duration of treatment benefit was 5 years, in a sensitivity analysis, we allowed the treatment benefit period to vary from 3 to 7 years by 1-year intervals. Additionally, the base-case model assumed that the overall survival treatment effect translated fully, with 100% effectiveness, to the corresponding cancer treatment population defined by the trial eligibility criteria. However, the benefits of positive trials may not fully generalize to all patient groups, suggesting that the efficiency with which the efficacy estimate translates to effectiveness in the cancer population may be < 100%.12 Conversely, newly proven treatments are commonly used off-label in populations of patients not represented in the trial.11 Thus, we allowed this efficiency parameter to vary from 80% to 120% by 10% intervals. Finally, the base case assumed uptake of the new treatment occurred the same year as the primary trial publication. However, uptake of new treatments in the cancer treatment community may precede publication of the article reporting the main trial results, especially if previously presented at a major cancer conference, or uptake may not be immediate (especially for medically disadvantaged patient groups).13 We allowed the year of adoption to uniformly vary from 2 years before trial publication to 2 years after trial publication by 1-year intervals.
