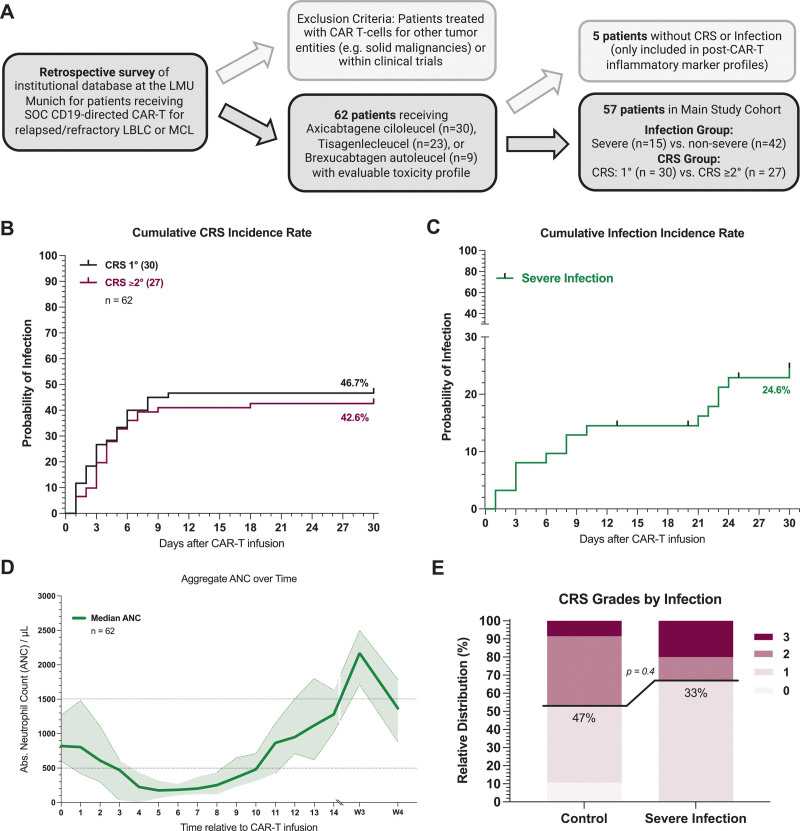
Figure 1.
CRS and infection rates in a real-world cohort of CAR-T–treated B-NHL patients. (A) Schematic overview of the study cohort with key inclusion and exclusion criteria. (B) Cumulative incidence rate of CRS grade 1 (gray) and CRS grade ≥2 (magenta) in the first 30 d after CAR-T infusion. (C) Cumulative incidence rate of severe infection (green) in the first 30 d after CAR-T infusion. Severe infections were defined as requiring i.v. anti-infective therapy and/or hospitalization and microbiologic evidence of infection. In the absence of microbiologic evidence overwhelming evidence of clinical infection had to present (eg, clinical symptoms and concordant imaging findings. (D) Aggregated median ANC over time for 62 patients between day 0 (CAR infusion) and day 30. Light shading depicts the 95% CIs of the median for each time point. (E) Relative distribution of CRS grades according to ASTCT grading in patients with severe infection (n = 15) and without severe infection events (n = 47). B-NHL = B-cell non-Hodgkin lymphoma; CAR-T = chimeric antigen receptor T-cell therapy; CI = confidence interval; CRS = cytokine release syndrome.