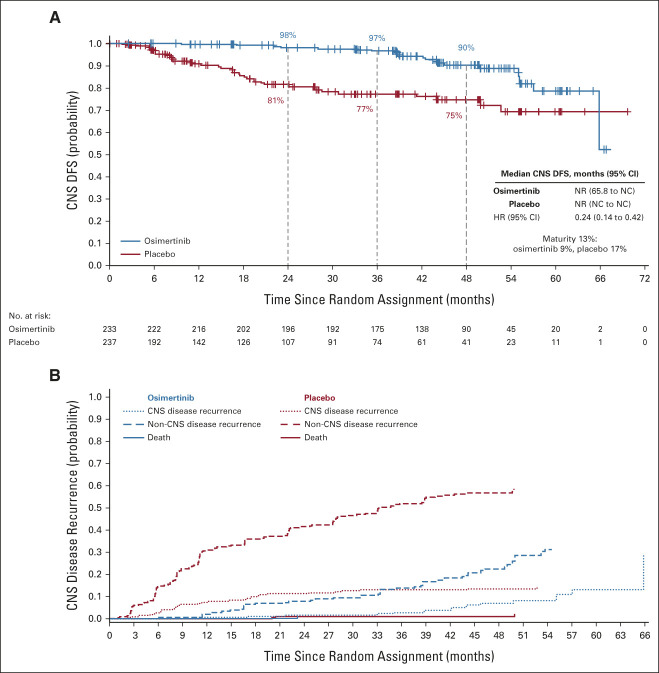
FIG 3.
CNS analyses (full analysis set; stage II-IIIA). Kaplan-Meier estimates of duration of (A) CNS DFS per investigator assessment in patients with stage II-IIIA disease. Tick marks indicate censored data. An HR < 1 favors osimertinib. (B) Conditional probability of observing CNS and non-CNS recurrence. The graph shows the estimated probability of observing CNS recurrence event, conditional on the patient not experiencing a competing risk event (non-CNS recurrence and death by any cause) by time t. Cumulative incidence was calculated using a Fine and Gray model. CNS disease recurrence includes patients who have disease recurrence in the CNS alone or in the CNS in addition to other anatomies at the same overall visit. Non-CNS recurrence includes disease recurrence outside the CNS only. Death was defined as death occurring without confirmed CNS or non-CNS recurrence. DFS, disease-free survival; HR, hazard ratio; NC, not calculated; NR, not reached.