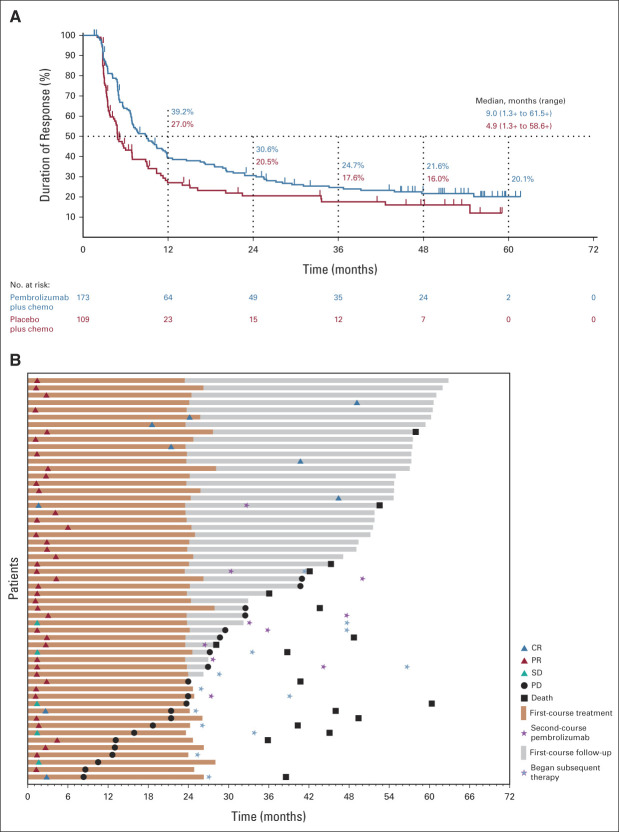
FIG 2.
Patient response to pembrolizumab plus chemotherapy and placebo plus chemotherapy. (A) DOR in the ITT population and (B) time to response and DOR in patients who completed 35 cycles of pembrolizumab. Median PFS was NR (95% CI, 21.2 months to NR) among patients who completed 35 cycles. The PFS rate 3 years after completion of 35 cycles was 58.4% (95% CI, 39.8 to 73.0). chemo, chemotherapy; CR, complete response; DOR, duration of response; ITT, intention to treat; NR, not reached; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.