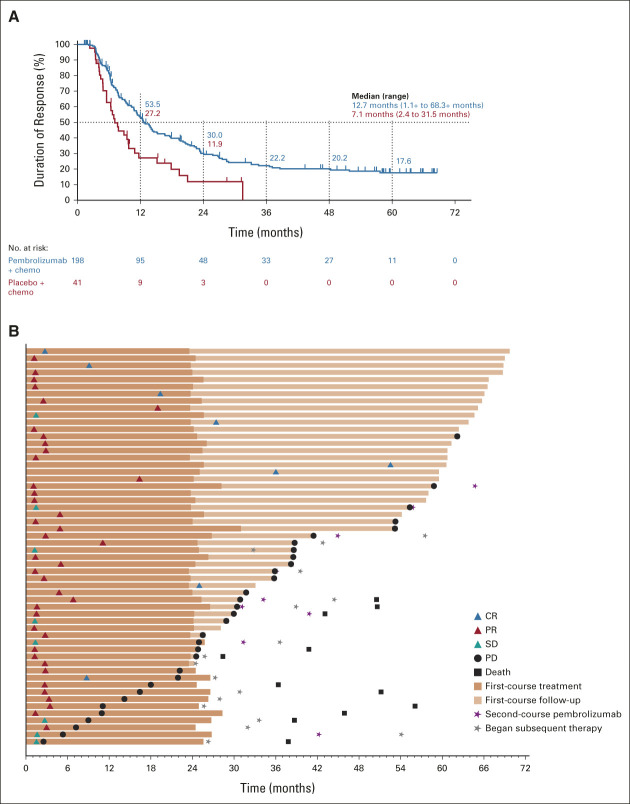
FIG 2.
(A) Duration of response in the ITT population and (B) treatment duration and time to response in patients who completed 35 cycles of pembrolizumab. Response assessments are shown per RECIST version 1.1 by BICR. Median PFS was not reached (95% CI, 14.7 months to not reached). PFS rate 3 years after completion of 35 cycles was 56.2% (95% CI, 39.7 to 69.8). BICR, blinded independent central review; chemo, chemotherapy; CR, complete response; ITT, intention-to-treat; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.