Abstract
As survival after pediatric intensive care unit (PICU) admission has improved over recent years, a key focus now is the reduction of morbidities and optimization of quality of life for survivors. Neurologic disorders and direct brain injuries are the reason for 11–16% of admissions to PICU. In addition, many critically ill children are at heightened risk of brain injury and neurodevelopmental difficulties affecting later life, e.g., complex heart disease and premature birth. Hence, assessment, monitoring and protection of the brain, using fundamental principles of neurocritical care, are crucial to the practice of pediatric intensive care medicine. The assessment of brain function, necessary to direct appropriate care, is uniquely challenging amongst children admitted to the PICU. Challenges in assessment arise in children who are unstable, or pharmacologically sedated and muscle relaxed, or who have premorbid abnormality in development. Moreover, the heterogeneity of diseases and ages in PICU patients, means that high caliber evidence is harder to accrue than in adult practice, nonetheless, great progress has been made over recent years. In this ‘state of the art’ paper about critically ill children, we discuss 1) patient types at risk of brain injury, 2) new standardized clinical assessment tools for age-appropriate, clinical evaluation of brain function, 3) latest evidence related to cranial imaging, non-invasive and invasive monitoring of the brain, 4) the concept of childhood ‘post intensive are syndrome’ and approaches for neurodevelopmental follow up. Better understanding of these concepts is vital for taking PICU survivorship to the next level.
Introduction
A key goal of care in the pediatric intensive care unit (PICU) is to achieve survival that ultimately leads to the fulfillment of neurodevelopmental potential. To protect the brains of critically ill children, as is necessary to achieve best possible outcomes, we need optimal evidence-based methods to assess it and to track neurodevelopmental function over the course of recovery. Such assessments are essential to guide treatment and care to protect the brain during the acute phase of illness, and then promote subsequent rehabilitation, as children return to normal life. The evolution of expertise in this field arose from diverse sources that span the age range. These include the experience from neonatal intensive care for hypoxic-ischemic encephalopathy (HIE) that translate to the vulnerable neonatal brain in related scenarios such as pediatric cardiac surgery[1, 2] and multisystem disorders special to the PICU such as metabolic encephalopathy[3]; lessons learned from adult stroke,[4] cardiac arrest,[5] and neurotrauma care;[6] and from a range of bespoke studies set in the PICU that we reference specifically in the sections of this article. We discuss unique aspects of assessment, monitoring, investigations, and follow-up of the brain in pediatric critical care, focusing on 1) clinical groups in PICU with vulnerable brains, 2) bedside clinical assessment of neurological function, 3) non-invasive monitoring and imaging, invasive monitoring and, 4) the concept of post intensive care syndrome[7] and follow up entailing coordination with specialists, outside our specialty, to seek out best environmental enrichment and rehabilitation strategies that have the potential for improving the natural history post injury brain maturation.
Section 1: Vulnerable Brains in PICU
Brain assessment and its integration into pediatric critical care management is a cornerstone of care for children with primary neurological conditions or direct brain injury and the significant proportion of critically ill children at risk of secondary brain injuries. As an illustration of scale, we know that out of a total pediatric population in the UK of around 14 million, 11% of the 60,000 PICU admissions between 2017 to 2019, had a neurologic disorder, brain injury or nervous system morbidity.[8] In the Prevalence of Acute critical Neurological disease in children: a Global Epidemiological Assessment (PANGEA) point prevalence study, set in 109 hospitals predominantly in North America and Europe, 16.2% of children in the PICU were affected by an acute primary neurological condition, the most common being cardiac arrest and severe traumatic brain injury (sTBI).[9] An epidemiological study of PICU admissions in Australia and New Zealand found that amongst 103,367 admissions, 14.4% had a primary neurological diagnosis [10] (e.g., sTBI, neuro-infection or inflammation, status epilepticus, stroke and hypoxic ischemic brain injury[11]). In addition, a significant proportion of PICU admissions although not suffering from primary neurologic disease, are nonetheless at risk of secondary mechanisms of injury, such as the 2.8% with severe sepsis [3]. Last, there is the 20% risk of morbidity in the PICU population with cardiac disease or those seen after congenital heart surgery, with multiple interacting risk factors: abnormal in utero brain development [1]; underlying genetic abnormalities affecting cerebral structures [12]; cardiopulmonary bypass related acquired brain injury; and, inadequate peri-operative brain perfusion during post-operative low cardiac output states.[2]
Critically ill children with these complex multi-system conditions require a range of assessment and monitoring to guide the care, including electroencephalographic (EEG) staging of encephalopathy, brain imaging tailored, use of invasive intracranial pressure (ICP) measurement, and meticulous attention to each organ system derangement. This strategy is seen in our current approach to multisystem problems in, for example: the infant with hyperammonemia and presumed metabolic disorder needing extracorporeal renal support;[3] the child with super refractory status epilepticus in a category of so-called febrile infection-related epilepsy syndrome undergoing anesthesia and immunomodulation for management;[13] and, the older patient seen after hematopoietic stem cell transplant with disseminated viral infection, coagulopathy, cardiac depression and acute-on-chronic lung injury.[14] The complexity and diversity of these clinical scenarios demonstrates why several approaches to brain assessment are required and integrated into the patients journey and care.
Section 2: Clinical assessment of neurological function in PICU
Clinical neuromonitoring is the process of serial neurologic examination in critically ill patients. Such assessment is invaluable in children with primary neurological conditions or direct brain injury, as well as those with neurological complications of systemic diseases. Available instruments used at the bedside are shown in Table 1 and described below.
Table 1:
Tools for Clinical Assessment of Brain Function in PICU
| Assessment Tool | Domains included | Intended patients | Who administers | Practical issues |
|---|---|---|---|---|
| Pediatric modification of Glasgow Coma Scale (GCS)[21] | Global assessment of neurologic function
|
Scales for patients ≥2 and <2 years old |
Nurse Advanced Nurse Practitioner Physician |
|
| Serial neurologic assessment in pediatrics (SNAP)[24] | Global assessment of neurologic function
|
Scales for patients ≥2 years old, <2 years and ≥6 months old, and <6 months old |
Nurse Advanced Nurse Practitioner Physician |
|
| Cornell Assessment of Pediatric Delirium (CAPD)[29] | Assessment for delirium | All patients <18 years old | Nurse Advanced Nurse Practitioner Physician |
|
| Pediatric Confusion Assessment Method for the ICU (pCAM-ICU)[33] Preschool version of pCAM-ICU (Ps-CAM-ICU)[31] |
Assessment for delirium | Ages >5–18 years Ages >6 mo-5 years |
Nurse Advanced Nurse Practitioner Physician |
|
| Pediatric delirium component (PD-scale) of the Sophia Observation Withdrawal Symptoms scale (SOS-PD scale)[32] |
Assessment for delirium | Ages >3 mo-18 years | Nurse Advanced Nurse Practitioner Physician |
|
Clinical evaluation of brain function in PICU
Clinical monitoring, whether intermittent or serial, [15, 16] has the potential to identify deficits representing new or evolving direct nervous system injury. In practice, however, the key to performing the neurologic examination is observation, as confrontational examinations can be challenging, especially in children with developmental disabilities and those who are intubated and/or sedated. A common initial manifestation in children is encephalopathy (i.e., irritability, crying, sleepiness, or agitation), or seizures rather than focal deficits. Then, there is the problem of serial examinations by multiple assessors, such as physicians, nurses, therapists, and parents.[17] Here, in common with adult neurocritical care practice, there is the obvious need for a standardized screening tool to improve detection of clinically relevant neurologic changes, e.g., the Glasgow Coma Scale (GCS), Alert Voice Pain Unresponsive (AVPU), and Full Outline of UnResponsiveness (FOUR).[18, 19] Pediatric modifications of these scoring systems have been developed for children,[20–22] and even though scores such as the GCS are used in most PICUs, limitations impede the ability to reliably detect changes in a critically ill child’s neurologic examination.[17, 18, 23]
The new Serial Neurologic Assessment in Pediatrics (SNAP) tool was developed to optimize screening neurologic assessments in children.[24] It was designed for contemporary PICU practice that includes children who are intubated, sedated, and/or have developmental disabilities. SNAP assesses mental status, cranial nerves, communication, and motor function. When used by PICU nurses, SNAP had substantial to near-perfect interrater reliability and is feasible to implement.[24] When standardized reporting of changes detected on a screening assessment is communicated to physician teams, it may lead to further diagnostics, earlier identification of neurologic injury, and management decisions directed at preventing or mitigating irreversible brain injury or death.
Delirium evaluation
In addition to identifying direct neurologic injury, it is also important to identify acute brain dysfunction that arises as a secondary complication of systemic disease. Delirium is a behavioral syndrome,[25] defined as an acute and fluctuating change in mental status, due to three possible (and synergistic) etiologies: underlying illness, treatment side effects, and/or as a reaction to the disruptive PICU environment. Delirium affects approximately one in four critically ill children, and is linked to poorer PICU outcomes [26, 27]. Without routine screening, delirium often goes undetected in its early stages[26]. Therefore, guidelines recommend routine delirium screening each shift as standard of care, throughout a child’s PICU stay.[27, 28] The Cornell Assessment of Pediatric Delirium (CAPD), the most widely used pediatric tool, is an observational scale scored by bedside nurses. A score of nine or higher is consistent with the gold-standard psychiatric diagnosis of delirium.[29] A modified scoring algorithm has been developed for children with significant underlying developmental disabilities to improve delirium identification in this challenging population.[30] The CAPD has been validated in children of all ages and developmental stages, has excellent interrater reliability, and is available in more than a dozen languages. The Pediatric Confusion Assessment Method for the ICU (pCAM-ICU) is used in children older than 5 years, and the accompanying preschool version (ps-CAM-ICU) for children under 5 years.[31] These are interactive screening tools that provide a point-in-time assessment for delirium. In addition, the Sophia Observation Withdrawal Symptoms Scale (SOS) has an extended pediatric delirium (PD) component (SOS-PD) that can be used to screen for delirium.[32] It consists of 18 items scored by the bedside nurse. Either the CAPD, p/psCAM-ICU, or SOS-PD can be used to screen for delirium, based on center preference for an observational versus interactive tool.
Section 3: Pediatric neurocritical care monitoring and clinical implications
Brain imaging in PICU
Neuroimaging offers valuable insights into characterisation, aetiology, response to therapy and prognostication of neurological disease in critically ill children but must always be interpreted within the context of the clinical history and findings. There are no evidence-based guidelines for neuroimaging in the PICU, and decision-making as to which scan is appropriate at specific timepoints in the patient journey is multi-disciplinary and guided by the patient’s condition.
Portable cranial ultrasound (CUS) offers bedside diagnosis in critical unstable children, for example to rule out intracranial haemorrhage prior to initiation of extracorporeal membrane oxygenation (ECMO), but is inadequate for use in prognostication and is less sensitive than computed tomography (CT) and magnetic resonance imaging (MRI).[34] To increase yield, contrast enhanced ultrasound (microbubbles of ultrasound contrast) is a new technique for real-time perfusion in neonates,[35] [36] including after cardiac surgery.[37] Nonetheless CUS is used frequently as part of the bedside assessment of unstable neonates and infants with open anterior fontanelle following major events at any timepoint in their admission.
Early CT (≤24 hours) in children with suspected hypoxic, ischaemic or sTBI, helps exclude haemorrhage, may indicate severity of hypoxic ischaemic injury and with the use of CT angiography, and can resolve neck and intracranial arterial anatomy. The established indices in adults of early ischaemic changes of grey to white matter attenuation ratio (GWR), and the modified Alberta Stroke Program Early CT Score (mASPECTS), have shown initial utility for prognostication in children in the contexts of extracorporeal cardiopulmonary resuscitation and other types of cardiac arrest.[38, 39] However the GWR should be interpreted in the context of serial clinical assessments and monitoring such as the results of the EEG.
In neonates, neurological injury may be subclinical or undetectable early on, and CUS may not detect subtle abnormalities, particularly in posterior cerebral lobes and cerebellum. Then, given that there is lower contrast resolution on CT, since neonates and infants have unmyelinated brains, MRI brain is considered the best imaging modality for characterisation and prognostication of brain injury in PICU. MRI diffusion weighted imaging (DWI) has an important role in the diagnosis of acute brain damage including arterial ischemic events during the acute phase, however diffusion changes start to disappear after 5 days (pseudo-normalization) and the timing of DWI changes may be influenced by the use of cooling.[40] With neonatal HIE, MR spectroscopy shows changes within the first 24 hours when even the DWI can be negative.[41] The optimal time for DWI is between 1 and 5 days, at which point the injury may be timed and characterized. Then, after day 5, chronic changes in T2 and T1 weighted images may be helpful in prognostication.
Non-invasive neuromonitoring in PICU
Non-invasive neuromonitoring is used broadly in PICUs: a recent US survey showed that all surveyed institutions had EEG monitoring capabilities, with 96% using continuous EEG, 87% using near infrared spectroscopy (NIRS) and 40% using transcranial Doppler (TCD), with other non-invasive monitoring capabilities (e.g., pupillometry, optic nerve sheath diameter [ONSD], bispectral index [BIS] monitor) used more sparingly.[42] The conclusion from the adult focussed International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care, that advanced analytical methods applied to multimodality monitoring of focal and global neurophysiologic cerebral alterations would enhance assessments compared to clinical examinations only,[43] is currently being considered and adapted by PICU groups with age specific adjustments (Table 2).
Table 2:
Non-invasive Neuromonitoring Methods Currently Used or Under Investigation in Pediatric Neurocritical Care
| Method (application in PICU) | Intended Utility | Suggested thresholds | Disadvantages |
|---|---|---|---|
| Electroencephalography (EEG represents standard of care in children clinically at risk of brain injury and seizures – established)[44] |
Detection of seizures (electro-clinical and electrographic-only) Diagnosis of non-convulsive status Prognostication after hypoxic brain insult |
High risk neonates and children (with hypoxic brain insult, stroke, infection, head trauma, inborn error of metabolism, clinically suspected seizures/epilepsy) | Limited availability in resource limited settings. |
| Near infrared spectroscopy (Guidelines are widely used in neonatal cardiac surgery - established, there is limited experience in other patient groups – used less often, under investigation)[53] |
Regional cerebral tissue oxygenation | More than 20% decline from baseline rSO2 <50% rSO2 <40% Left-to-right difference in rSO2 of >10% |
Limited spatial resolution No evidence for outcome improvement |
| Transcranial Doppler (Consensus based guidelines exist for use in PICU although the evidence base is limited– used less often, under investigation)[57] |
Cerebral blood flow velocity monitoring | Age dependent | Inter-operator variability |
| Non-invasive intracranial monitoring | Poor accuracy for intracranial monitoring compared to invasive methods | ||
| Automated pupillometry (Occasionally used clinically, research tool, the evidence base is weak – under investigation)[65] |
Assessment of pupillary size, asymmetry, constriction to light, latency, constriction and dilation velocity | Diameter <0.5 mm Asymmetry <0.5 mm %Pupillary light response 35%−40% Constriction velocity 1.5 mm/sec Dilation velocity 2.83 mm/sec Neurological pupillary index ≥3 |
Limited data on effects of sedatives and other medications on pupillary reactivity |
| Optic nerve sheath diameter (Occasionally used clinically, research tool, the evidence base is weak– under investigation)[66] |
Non-invasive intracranial pressure measurement | Optic nerve sheath diameter >5 mm as indicator of intra cranial pressure >20 mmHg Age dependent |
May be used to detect increased intracranial pressure shadowing artifact |
Electro encephalography (EEG)
Seizures are the most common neurological emergency in critically ill neonates and children, and EEG is required for their accurate diagnosis. Due to growing awareness about the high prevalence of subclinical seizures among patients with acute encephalopathy, brain injury or clinical seizures, continuous EEG monitoring has become the standard of care for selected critically ill neonates and children in high-resource centres. Common indications for continuous EEG include hypoxic or traumatic brain injury, stroke, refractory seizures, meningoencephalitis, and unexplained encephalopathy (Table 2) [44, 45] Observational cohort studies in both neonates and children have demonstrated that higher seizure burden is associated with worse short- and long-term outcomes, even after adjusting for other factors such as brain injury aetiology and severity.[46, 47] Yet the question remains to what extent seizures independently cause secondary brain injury and worse outcomes. The potential deleterious effects of seizures likely depend on seizure aetiology (e.g.: acute stroke versus, epilepsy), seizure type (e.g.: focal versus. generalized) and other factors such as age and concomitant brain injury. Randomized controlled trials (RCT) in neonates of EEG-guided seizure treatment compared with treatment of clinical seizures alone demonstrated that EEG-guided therapy successfully reduced overall seizure burden; however, potentially related to poor sample size, RCTs failed to demonstrate a difference in outcome between the treatment groups[48], [49] Nonetheless, implementation of continuous EEG monitoring can improve the timeliness of seizure detection and seizure control, and that this in turn is associated with decreased use of anti-seizure medications both in hospital and upon discharge, and less frequent progression to status epilepticus.[50, 51] Finally, in addition to providing insights on seizure burden, continuous EEG can provide valuable insights into prognosis through ongoing assessment of EEG background activity. Severe background suppression, or a burst suppression pattern, especially if invariant and prolonged, in the absence of confounding factors such as sedative medications portend a poor outcome.[52]
Near infrared spectroscopy (NIRS)
NIRS-based cerebral regional tissue oxygenation (rSO2) is used widely in children on ECMO and in children admitted to the PICU following cardiac surgery, in particular complex neonates who are at high-risk of brain injury, and is reported as standard of care in many high resource settings despite an absence of RCTs.[53] Management algorithms have been published, only for infants and children with congenital heart disease in the PICU, suggesting cerebral rSO2 thresholds (>20% decline from baseline cerebral rSO2, cerebral rSO2 <50% or <40%, left-to-right difference in cerebral rSO2 >10%) to trigger interventions intended to avert severe low cardiac output states, linked to secondary brain injury.[53] NIRS-based cerebrovascular autoregulation monitoring has been studied in children admitted to PICU following cardiac surgery,[54] sTBI,[55] and cardiac arrest.[56] Although it has been speculated that goal directed hemodynamic management that targets an optimal mean arterial pressure for preservation of autoregulation, using NIRS-based monitoring, could be linked to better neurodevelopmental outcome, this is as yet unproven.
Transcranial doppler (TCD)
TCD measures cerebral blood flow velocity in large cerebral vessels and is a useful tool in critically ill children with concern for pathophysiological changes in cerebrovascular hemodynamics.[57] In critically ill children with pathologies as varied as sTBI, arterial ischemic stroke, hydrocephalus, bacterial meningitis, or diabetic ketoacidosis, TCD has been utilized to noninvasively estimate ICP,[58] to serially monitor for presence and severity of vasospasm,[59] and to assess cerebrovascular autoregulation.[58] Other TCD applications in adult neurocritical care have either been extremely rarely used in the PICU or have not yet been reported (i.e., evaluation of arteriovenous malformations or of collateral pathways of intracranial blood flow, as adjunct to brain death evaluation, and in the assessment of cerebral microemboli, right-to-left shunts, ICP, hydrocephalus, hypoxic ischemic encephalopathy, or dural venous sinus patency).[58] Pediatric-specific standards for technical performance, data interpretation, and data reporting standards have recently been published in an effort to ensure reproducibility between TCD operators and across institutions.[57]
Pupillometry
Automated pupillometry is gaining ground in clinical practice, primarily due to increased recognition of improved accuracy over manual assessment.[60] Normative data have been published for healthy paediatric volunteers,[61] but data in critically ill children are extremely limited. A recent single-centre prospective observational study conducted in 28 children admitted to the PICU with acute brain injury or encephalopathy requiring an ICP monitor showed that percent change in pupillary size, constriction velocity, dilation velocity, and Neurologic Pupil index (NPi) were lower when ICP was ≥20 mm Hg versus <20 mm Hg (among 1,171 concomitant automated pupillometry and ICP measurements).[62] Abnormal pupillary measurements were only associated with concurrent and not future ICP measurements.[62] Potential future uses of pupillometry may include the assessment and optimization of analgesic regimens for children in PICU[63] and prognostication, although this would be contingent on further research to understand the role of potential confounders.[64]
Invasive monitoring
The latest international consensus guidance,[67] recommends invasive ICP monitoring in children with sTBI (usually in children with GCS<9 with trauma related abnormal CT scan) to allow titration of therapies in a tiered fashion. Insertion of intra-parenchymal catheter via a bolt is common due to ease of insertion, performance and use, though the catheters can also be placed elsewhere. Treatment pathways support keeping the ICP <20mmHg and achieving a minimum cerebral perfusion pressure (CPP) at least 40–50 mmHg.[67] This practice is supported by observational data from critically ill adults.[68] Observational data from children with sTBI indicates that prolonged and intense rises in ICP are adverse, with poor tolerance in young age groups, implying these require careful management.[69] Very young children with sTBI may be at heightened risk of impaired cerebrovascular pressure autoregulation, with potential to benefit from individualized treatment targets for management of cerebral perfusion pressure.[70] A prospective hybrid implementation and effectiveness study (PEGASUS) in pediatric sTBI indicated that adherence to cerebral perfusion pressure targets, avoidance of hypocarbia and adequate enteral nutrition were all linked to better hospital survival.[71] Intracranial hypertension is a major cause of morbidity and mortality in various non-traumatic encephalopathies that affect children (e.g. hypoxic ischaemic encephalopathy, stroke, sinus venous thrombosis, fulminant hepatic failure, diabetic ketoacidosis, meningo-encephalitis, brain tumours and idiopathic intracranial hypertension) and invasive monitoring can offer guidance for reducing secondary insults.[72] However, since such monitoring has limited links with outcome, invasive monitoring is used sparingly in these conditions In the example of bacterial meningitis in PICU, risk stratification tools have shown the potential to identify where invasive monitoring benefits outweigh the risks through personalised treatment pathways.[73] There is insufficient evidence to recommend the use of partial pressure of brain tissue oxygen (PbrO2) monitoring.[67] Though, if such monitoring is used, a minimum value of 10mmHg is supported.[67] Strategies to improve PbrO2 may include manipulation of CPP, haemoglobin, FiO2, and CO2. PbrO2 monitoring may also be used to monitor for evidence of cerebral ischaemia if hyperventilation is used as a treatment strategy for raised ICP. Cerebral autoregulation-based derivation of optimum CPP using correlation between ICP and blood pressure measurements, as well as cerebral microdialysis based analysis of lactate/pyruvate ratio, glucose, glutamate etc. are currently limited to research settings only.
Section 4: The brain following PICU discharge
Post Intensive Care Syndrome (PICS-p)
The figure depicts the recently conceptualized ‘Post-Intensive Care Syndrome in Pediatrics (PICS-p)’,[7] which describes how new or worsening morbidities in PICU survivors can align to physical, cognitive, emotional and social health domains. PICS-p recognizes that both the child’s pre-existing health status, the management of their acute critical illness, and their stage of maturation and growth, may affect the severity and pervasiveness of acquired impairments beyond the PICU. Furthermore, the framework identifies the PICU survivors are part of a family unit, where family members such as caregivers and siblings may also be affected. For example, both children and caregivers may experience psychological responses such as post-traumatic stress (PTS)[74] and psychological burdens may contribute to social and emotional recovery.[75]
Figure:
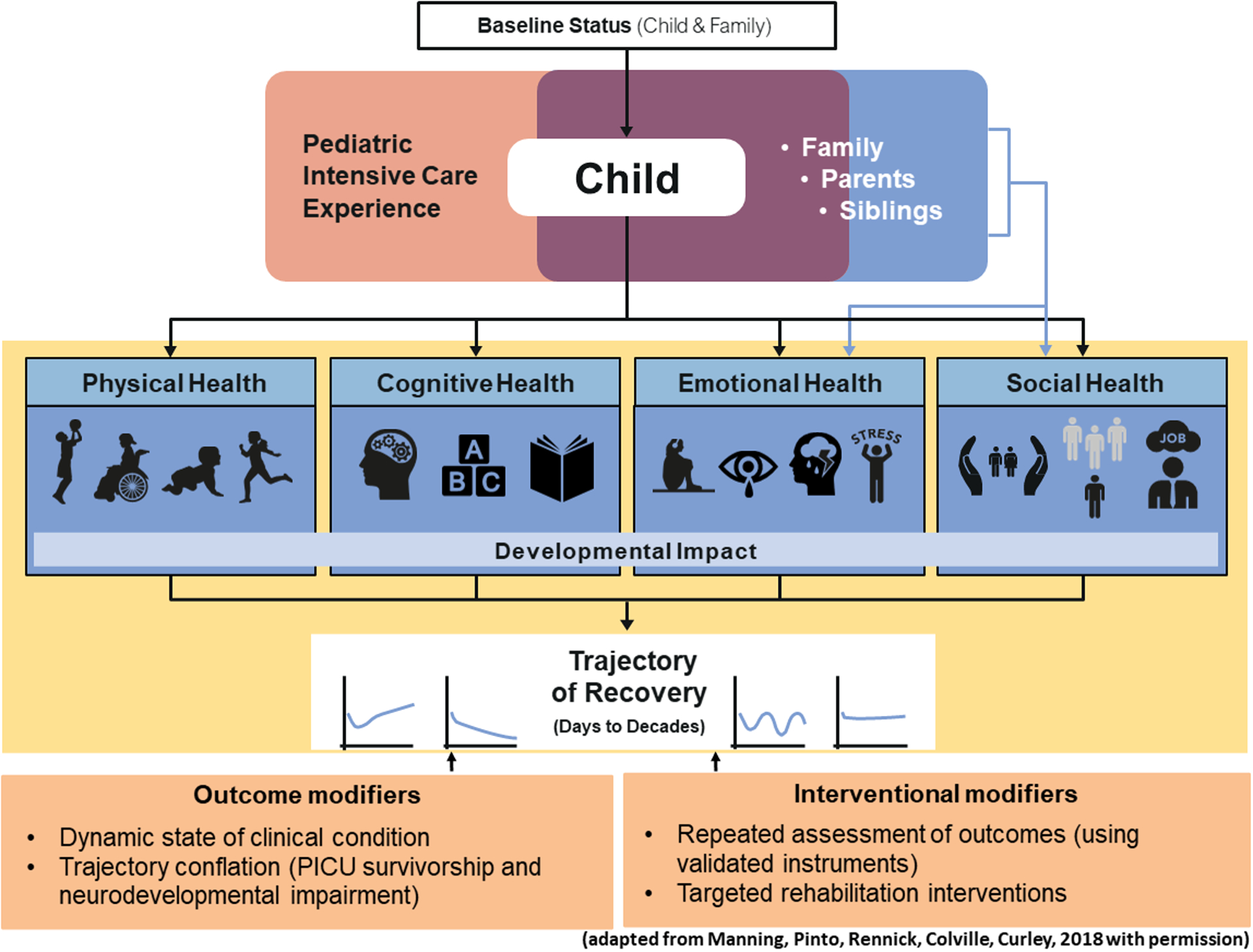
The figure depicts the recently conceptualized ‘Post-Intensive Care Syndrome in Pediatrics (PICS-p)
Within the physical domain, outcomes of critical illness may be organ specific or more generalized including respiratory dysfunction, chronic pain, epilepsy, sleep disruption, fatigue, severe muscle weakness, reduced self-care and feeding disturbance[76]. Physical adverse outcome may be due to the underlying illness (e.g.: hearing loss after meningitis), due to PICU treatment (e.g.: subglottic stenosis after endotracheal intubation) or due to the combination of both. Clearly, brain injuries and neurological conditions may affect cognitive function and children’s social and emotional development. The physical, cognitive, emotional, and social recovery which run simultaneously, starting at PICU discharge, and concluding in reaching a ‘new normal’, captured within the concept of health-related quality of life (QoL). A review of QoL following PICU admission concluded that worse scores related to pre-existing conditions, PICU interventions and events (e.g.: ECMO or cardiac arrest), social and environmental factors and parent mental health.[77] The concept of PICS-p helps us to contextualize ND outcomes after PICU admission by providing a framework that recognizes the complex and multifaceted nature of survivorship and frames short- and longer-term outcomes following childhood critical illness as holistic, dynamic, and inter-relational.
Neurodevelopmental (ND) follow-up programs and pathways
The provision of ND follow-up after PICU is better established in some regions, whereas elsewhere this is an emerging area of health care need that is yet to be developed. Although standards of care vary, there is little debate that children with confirmed brain injury (e.g.: sTBI, post-cardiac arrest, stroke, central nervous system infection, seizure disorders) require structured follow up. In most high resource settings, ND follow up is also recommended for children who are at high risk of brain injury, even if this is not identified at the point of discharge, for example, ex-premature infants and children with complex congenital heart disease. When considering whether or not ND follow-up is required for specific children post PICU, the following risk features may be considered:
Pre-existing factors of the child and family: e.g.: medical history of high risk conditions or co-morbidities, difficult socioeconomic status, and young age at the time of a critical event, which makes initial assessment less reliable.[78] [79]
Management during and characteristics of the PICU admission: e.g.: high severity of illness, emergent nature of the admission, prolonged PICU stay, prolonged mechanical ventilation, specific treatments such as ECMO and presence of PICU-acquired delirium.[79] [80]
Recovery phase after PICU discharge: e.g.: anxiety and stress levels of the child and parent, coping strategies within the family, readmissions to the hospital, rehabilitation needs of the child.[79]
Follow-up assessments should take place into early adulthood, given the importance of understanding and monitoring the impacts on long-term functioning, basic daily skills and education, and considering that children may grow into deficits. PICU follow-up needs to be a joint venture between the relevant practitioners and be delivered by the best available combination of professionals equipped to provide it, which varies based on local health care systems. This might include: PICU physicians, child development and neurology subspecialists, primary care providers, community pediatricians, nurses, physiotherapists, psychologists and if applicable palliative care specialists, who ideally should follow standardized follow-up intervals and use well validated instruments that have internationally normative data. Commonly used measures that cover most relevant domains and attributes are displayed in Table 3, noting their main limitations include: lack of precision (e.g. broad category descriptions); subjective scoring criteria and measurements methods; and failure to capture important functional problems, including sleep disruption, fatigue, and severe muscle weakness.[81] Of note all the measures listed have specific age appropriate versions and instructions for use.
Table 3:
Physical, Psychosocial, Neurocognitive and Quality of Life Outcomes after PICU Admission
| Outcome or Parameter | Assessment in | By whom | When | How (example) |
|---|---|---|---|---|
| Physical outcomes | ||||
| Clinical neurological evaluation | Child | Nurse Advanced Nurse Practitioner Physician |
Hospital discharge and follow up and at least 6 months later |
|
| Health status, functional status | Child | Nurse Advanced Nurse Practitioner Physician |
Hospital discharge and follow up and at least 6 months later |
|
| Readmissions | Child | Parent/Physician | As applicable | |
| Motor development | Child | Physiotherapist | At least 6 months post discharge |
|
| Perception/sensation | Child | Physician | ||
| Psychosocial outcomes | ||||
| (Adaptive) Behaviour | Child | Self and/or parent | At least 6 months post discharge |
|
| Family burden | Parent | Parent | At least 6 months post discharge |
|
| Emotions (regulation) | Child Parent |
Self and parent Parent |
At least 6 months post discharge |
|
| Coping | Child Parent |
Self and parent Parent |
At least 6 months post discharge |
|
| Social functioning | Child | Self | At least 6 months post discharge |
|
| Post-traumatic stress | Child Parent |
Psychologist Self and parent Parent |
At least 6 months post discharge and from age 7 years |
|
| Neurocognitive outcomes | ||||
| General intelligence | Child | Psychologist | At least 6 months after discharge and age 2 years |
|
| Attention | Child | Psychologist | At least 6 months after discharge and from age 6 years |
|
| Memory (visual/verbal) | Child | Psychologist | At least 6 months after discharge and from age 6 years |
|
| Visuo-motor integration | Child | Psychologist | At least 6 months after discharge and from age 2 years |
|
| Executive functioning | Child | Psychologist Parent |
At least 6 months after discharge and from age 2 years |
|
| Language development | Child | Psychologist | At least 6 months after discharge and from age 2 years |
|
| Quality of life | ||||
| Physical functioning | Child and Parent | Self and parent | At least 6 months post discharge |
|
| Emotional functioning | Child and Parent | Self and parent | At least 6 months post discharge |
|
| Social functioning | Child and Parent | Self and parent | At least 6 months post discharge |
|
There are major challenges in developing a ND follow-up program or facilitating a follow up pathway that is designed for use by a wide circle of linked health professionals outside the center where the PICU is located, for example the American Heart Association Guideline for the ND follow up of children with heart disease:[82]
Firstly, pediatric critical illness is extremely heterogeneous with respect to disease process and ages spanning widely different developmental stages. This means that a ND follow up program or pathway needs to be diverse, complex, flexible and multi-disciplinary, which can be difficult to achieve.
Secondly, if ND follow up data are to be used for future research, to improve consistency in reporting, outcomes should be based on a core outcome set (COS) to reduce heterogeneity and reporting bias, improve comparability and enable the pooling of data for meta-analyses. The recently developed PICU-COS emphasizes the domains of cognitive, emotional and physical function, overall health and QoL but has not yet established how these should be measured.[83] The methods and perceptions of these domains vary among health care professionals, caregivers and patients due to cultural aspects, beliefs and socio-economic factors, hence the next phase of identifying preferred standardized methods to evaluate these will be crucial.
Thirdly, a PICU admission also impacts parents, and parental outcomes are of great interest during follow up, especially since outcomes such as executive functioning and QoL in young children are often assessed by parents. Due to the shared variance between parents and children, the reported child outcomes might be colored by the subjective well-being of the parent. This indicates the importance of screening for emotional and psychological sequelae of PICU admission in both the child and their parents and focusing on family centered interventions and care.[84]
Finally, ideally it would be possible to identify and intervene on potentially modifiable factors, from the timepoint of the child’s admission in the PICU and onwards in their journey through ND follow up, with a goal of improving both immediate and long-term outcomes in critically ill children and their families. To achieve this necessitates a tight collaboration between research and clinical care through these stages in the patient’s journey, which requires considerable investment of expertise, resources and time. Few countries have achieved this however, it is an ideal to aim at, whilst considering a range of service designs and the current technical developments in electronic health care records to get there.
Conclusions
Despite the inherent challenges involved in the assessment of brain function and the conduct of neurocritical care for children, this field will only gain greater emphasis over time. As pediatric critical care practitioners it is essential that we continue to use every available opportunity, including learning from adult studies and designing-conducting high-quality pediatric studies in neurocritical care and entailing ND follow up. If we can rise to these challenges and prioritize learning about which PICU interventions are beneficial to brain health and/ or for optimizing later ND outcomes, we may see a step change in quality of life for survivors of childhood critical illness.
Take home message.
The practice of neurocritical care for children with injured or vulnerable brains entails clinical assessment, a range of monitoring methods within the PICU and the follow up of children’s long-term neurodevelopment. These activities involve inherent challenges related to the diversity of case-mix and age range. Nonetheless, the assessment of brain injury and brain function are vital both within PICU and later during follow-up, in order to take PICU survivorship to the next level.
Funding statement:
Research reported in this publication was supported by the National Institute of Health Biomedical Research Center at Great Ormond Street Hospital, London UK and the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R01NS106292 (Dr. Bembea). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
COI Statement: RP is an investigator for studies with UCB and does consultancy work for Kephala, Ireland. She served as a Speaker and/or on Advisory Boards for Natus, GW, Esai, and UCB. The other authors declare no conflict of interest.
References
- 1.Peyvandi S, Lim JM, Marini D, Xu D, Reddy VM, Barkovich AJ, Miller S, McQuillen P, Seed M, (2021) Fetal brain growth and risk of postnatal white matter injury in critical congenital heart disease. J Thorac Cardiovasc Surg 162: 1007–1014 e1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan I, Gaynor JW, Licht DJ, Loepke AW, (2021) Cutting the Gordian Knot That Ties Intraoperative Conditions to Long-term Neurodevelopmental Outcomes in Children Undergoing Congenital Heart Surgery. J Cardiothorac Vasc Anesth 35: 2889–2891 [DOI] [PubMed] [Google Scholar]
- 3.Raina R, Bedoyan JK, Lichter-Konecki U, Jouvet P, Picca S, Mew NA, Machado MC, Chakraborty R, Vemuganti M, Grewal MK, Bunchman T, Sethi SK, Krishnappa V, McCulloch M, Alhasan K, Bagga A, Basu RK, Schaefer F, Filler G, Warady BA, (2020) Consensus guidelines for management of hyperammonaemia in paediatric patients receiving continuous kidney replacement therapy. Nat Rev Nephrol 16: 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robba C, Bonatti G, Battaglini D, Rocco PRM, Pelosi P, (2019) Mechanical ventilation in patients with acute ischaemic stroke: from pathophysiology to clinical practice. Crit Care 23: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandroni C, Cronberg T, Sekhon M, (2021) Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med 47: 1393–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robba C, Poole D, McNett M, Asehnoune K, Bosel J, Bruder N, Chieregato A, Cinotti R, Duranteau J, Einav S, Ercole A, Ferguson N, Guerin C, Siempos II, Kurtz P, Juffermans NP, Mancebo J, Mascia L, McCredie V, Nin N, Oddo M, Pelosi P, Rabinstein AA, Neto AS, Seder DB, Skrifvars MB, Suarez JI, Taccone FS, van der Jagt M, Citerio G, Stevens RD, (2020) Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med 46: 2397–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ, (2018) Conceptualizing Post Intensive Care Syndrome in Children-The PICS-p Framework. Pediatr Crit Care Med 19: 298–300 [DOI] [PubMed] [Google Scholar]
- 8.PICANeT Paediatric Intensive Care Audit Network (2020) Annual Report. NHS Annual Report. LEEDS UK. [Google Scholar]
- 9.Fink EL, Kochanek PM, Tasker RC, Beca J, Bell MJ, Clark RS, Hutchison J, Vavilala MS, Fabio A, Angus DC, Watson RS, Prevalence of Acute critical Neurological disease in children AGEAI, (2017) International Survey of Critically Ill Children With Acute Neurologic Insults: The Prevalence of Acute Critical Neurological Disease in Children: A Global Epidemiological Assessment Study. Pediatr Crit Care Med 18: 330–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moynihan KM, Alexander PMA, Schlapbach LJ, Millar J, Jacobe S, Ravindranathan H, Croston EJ, Staffa SJ, Burns JP, Gelbart B, Australian, New Zealand Intensive Care Society Pediatric Study G, the ACfO, Resource E, (2019) Epidemiology of childhood death in Australian and New Zealand intensive care units. Intensive Care Med 45: 1262–1271 [DOI] [PubMed] [Google Scholar]
- 11.Williams CN, Piantino J, McEvoy C, Fino N, Eriksson CO, (2018) The Burden of Pediatric Neurocritical Care in the United States. Pediatr Neurol 89: 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ita M, Cisneros B, Rosas-Vargas H, (2021) Genetics of Transposition of Great Arteries: Between Laterality Abnormality and Outflow Tract Defect. J Cardiovasc Transl Res 14: 390–399 [DOI] [PubMed] [Google Scholar]
- 13.Sculier C, Barcia Aguilar C, Gaspard N, Gainza-Lein M, Sanchez Fernandez I, Amengual-Gual M, Anderson A, Arya R, Burrows BT, Brenton JN, Carpenter JL, Chapman KE, Clark J, Gaillard WD, Glauser TA, Goldstein JL, Goodkin HP, Gorman M, Lai YC, McDonough TL, Mikati MA, Nayak A, Peariso K, Riviello J, Rusie A, Sperberg K, Stredny CM, Tasker RC, Tchapyjnikov D, Vasquez A, Wainwright MS, Wilfong AA, Williams K, Loddenkemper T, pSerg, (2021) Clinical presentation of new onset refractory status epilepticus in children (the pSERG cohort). Epilepsia 62: 1629–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Pinto LN, Stroup EK, Pendergrast T, Pinto N, Luo Y, (2020) Derivation and Validation of Novel Phenotypes of Multiple Organ Dysfunction Syndrome in Critically Ill Children. JAMA Netw Open 3: e209271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM, (2017) Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatr Crit Care Med 18: e122–e130 [DOI] [PubMed] [Google Scholar]
- 16.Pollack MM, Holubkov R, Funai T, Berger JT, Clark AE, Meert K, Berg RA, Carcillo J, Wessel DL, Moler F, Dalton H, Newth CJ, Shanley T, Harrison RE, Doctor A, Jenkins TL, Tamburro R, Dean JM, Eunice Kennedy Shriver National Institute of Child H, Human Development Collaborative Pediatric Critical Care Research N, (2015) Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med 43: 1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschen MP, Snyder M, Winters M, Ichord R, Berg RA, Nadkarni V, Topjian A, (2018) Survey of Bedside Clinical Neurologic Assessments in U.S. PICUs. Pediatr Crit Care Med 19: 339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G, (2014) The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol 13: 844–854 [DOI] [PubMed] [Google Scholar]
- 19.Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL, (2005) Validation of a new coma scale: The FOUR score. Ann Neurol 58: 585–593 [DOI] [PubMed] [Google Scholar]
- 20.Kirkham FJ, Newton CR, Whitehouse W, (2008) Paediatric coma scales. Dev Med Child Neurol 50: 267–274 [DOI] [PubMed] [Google Scholar]
- 21.Kirschen MP, Snyder M, Smith K, Lourie K, Agarwal K, DiDonato P, Doll A, Zhang B, Mensinger J, Ichord R, Shea JA, Berg RA, Nadkarni V, Topjian A, (2019) Inter-Rater Reliability Between Critical Care Nurses Performing a Pediatric Modification to the Glasgow Coma Scale. Pediatr Crit Care Med 20: 660–666 [DOI] [PubMed] [Google Scholar]
- 22.Czaikowski BL, Liang H, Stewart CT, (2014) A pediatric FOUR score coma scale: interrater reliability and predictive validity. The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses 46: 79–87 [DOI] [PubMed] [Google Scholar]
- 23.Kornbluth J, Bhardwaj A, (2011) Evaluation of coma: a critical appraisal of popular scoring systems. Neurocrit Care 14: 134–143 [DOI] [PubMed] [Google Scholar]
- 24.Kirschen MP, Smith KA, Snyder M, Zhang B, Flibotte J, Heimall L, Budzynski K, DeLeo R, Cona J, Bocage C, Hur L, Winters M, Hanna R, Mensinger JL, Huh J, Lang SS, Barg FK, Shea JA, Ichord R, Berg RA, Levine JM, Nadkarni V, Topjian A, (2021) Serial Neurologic Assessment in Pediatrics (SNAP): A New Tool for Bedside Neurologic Assessment of Critically Ill Children. Pediatr Crit Care Med 22: 483–495 [DOI] [PubMed] [Google Scholar]
- 25.(2013) American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. American Psychiatric Association, Arlington, VA [Google Scholar]
- 26.Traube C, Silver G, Reeder RW, Doyle H, Hegel E, Wolfe HA, Schneller C, Chung MG, Dervan LA, DiGennaro JL, Buttram SD, Kudchadkar SR, Madden K, Hartman ME, deAlmeida ML, Walson K, Ista E, Baarslag MA, Salonia R, Beca J, Long D, Kawai Y, Cheifetz IM, Gelvez J, Truemper EJ, Smith RL, Peters ME, O’Meara AM, Murphy S, Bokhary A, Greenwald BM, Bell MJ, (2017) Delirium in Critically Ill Children: An International Point Prevalence Study. Crit Care Med 45: 584–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith HAB, Besunder JB, Betters KA, Johnson PN, Srinivasan V, Stormorken A, Farrington E, Golianu B, Godshall AJ, Acinelli L, Almgren C, Bailey CH, Boyd JM, Cisco MJ, Damian M, deAlmeida ML, Fehr J, Fenton KE, Gilliland F, Grant MJC, Howell J, Ruggles CA, Simone S, Su F, Sullivan JE, Tegtmeyer K, Traube C, Williams S, Berkenbosch JW, (2022) 2022 Society of Critical Care Medicine Clinical Practice Guidelines on Prevention and Management of Pain, Agitation, Neuromuscular Blockade, and Delirium in Critically Ill Pediatric Patients With Consideration of the ICU Environment and Early Mobility. Pediatr Crit Care Med 23: e74–e110 [DOI] [PubMed] [Google Scholar]
- 28.Harris J, Ramelet AS, van Dijk M, Pokorna P, Wielenga J, Tume L, Tibboel D, Ista E, (2016) Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: an ESPNIC position statement for healthcare professionals. Intensive Care Med 42: 972–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, Halpert S, Augenstein J, Sickles LE, Li C, Greenwald B, (2014) Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med 42: 656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur S, Silver G, Samuels S, Rosen AH, Weiss M, Mauer EA, Gerber LM, Greenwald BM, Traube C, (2020) Delirium and Developmental Disability: Improving Specificity of a Pediatric Delirium Screen. Pediatr Crit Care Med 21: 409–414 [DOI] [PubMed] [Google Scholar]
- 31.Smith HA, Gangopadhyay M, Goben CM, Jacobowski NL, Chestnut MH, Savage S, Rutherford MT, Denton D, Thompson JL, Chandrasekhar R, Acton M, Newman J, Noori HP, Terrell MK, Williams SR, Griffith K, Cooper TJ, Ely EW, Fuchs DC, Pandharipande PP, (2016) The Preschool Confusion Assessment Method for the ICU: Valid and Reliable Delirium Monitoring for Critically Ill Infants and Children. Crit Care Med 44: 592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ista E, van Beusekom B, van Rosmalen J, Kneyber MCJ, Lemson J, Brouwers A, Dieleman GC, Dierckx B, de Hoog M, Tibboel D, van Dijk M, (2018) Validation of the SOS-PD scale for assessment of pediatric delirium: a multicenter study. Crit Care 22: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith HA, Boyd J, Fuchs DC, Melvin K, Berry P, Shintani A, Eden SK, Terrell MK, Boswell T, Wolfram K, Sopfe J, Barr FE, Pandharipande PP, Ely EW, (2011) Diagnosing delirium in critically ill children: Validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med 39: 150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farhat A, Li X, Huet B, Tweed J, Morriss MC, Raman L, (2021) Routine Neuroimaging: Understanding Brain Injury in Pediatric Extracorporeal Membrane Oxygenation. Crit Care Med [DOI] [PubMed] [Google Scholar]
- 35.Squires JH, Beluk NH, Lee VK, Yanowitz TD, Gumus S, Subramanian S, Panigrahy A, (2021) Feasibility and Safety of Contrast-Enhanced Ultrasound of the Neonatal Brain: A Prospective Study Using MRI as the Reference Standard. AJR Am J Roentgenol: 1–10 [DOI] [PubMed] [Google Scholar]
- 36.Didier RA, Biko DM, Hwang M, Unnikrishnan S, Wozniak MM, Yusuf GT, Sridharan A, (2021) Emerging contrast-enhanced ultrasound applications in children. Pediatr Radiol 51: 2418–2424 [DOI] [PubMed] [Google Scholar]
- 37.Knieling F, Ruffer A, Cesnjevar R, Regensburger AP, Purbojo A, Dittrich S, Munch F, Neubert A, Meyer S, Strobel D, Rascher W, Woelfle J, Jungert J, (2020) Transfontanellar Contrast-Enhanced Ultrasound for Monitoring Brain Perfusion During Neonatal Heart Surgery. Circ Cardiovasc Imaging 13: e010073. [DOI] [PubMed] [Google Scholar]
- 38.Sperotto F, Saengsin K, Danehy A, Godsay M, Geisser DL, Rivkin M, Amigoni A, Thiagarajan RR, Kheir JN, (2021) Modeling severe functional impairment or death following ECPR in pediatric cardiac patients: Planning for an interventional trial. Resuscitation 167: 12–21 [DOI] [PubMed] [Google Scholar]
- 39.Tetsuhara K, Kaku N, Watanabe Y, Kumamoto M, Ichimiya Y, Mizuguchi S, Higashi K, Matsuoka W, Motomura Y, Sanefuji M, Hiwatashi A, Sakai Y, Ohga S, (2021) Predictive values of early head computed tomography for survival outcome after cardiac arrest in childhood: a pilot study. Sci Rep 11: 12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackburn E, D’Arco F, Devito A, Ioppolo R, Lorio S, Quirk B, Ganesan V, (2021) Predictors of motor outcome after childhood arterial ischemic stroke. Dev Med Child Neurol 63: 1171–1179 [DOI] [PubMed] [Google Scholar]
- 41.Ghei SK, Zan E, Nathan JE, Choudhri A, Tekes A, Huisman TA, Izbudak I, (2014) MR imaging of hypoxic-ischemic injury in term neonates: pearls and pitfalls. Radiographics 34: 1047–1061 [DOI] [PubMed] [Google Scholar]
- 42.Kirschen ML K; Balakrishnan B; Wainright M; Appavu B , (2021) A survey of neuromonitoring practices in North American Pediatric Intensive Care Units. Pediatric Neurology E Pub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, Diringer MN, Stocchetti N, Videtta W, Armonda R, Badjatia N, Boesel J, Chesnut R, Chou S, Claassen J, Czosnyka M, De Georgia M, Figaji A, Fugate J, Helbok R, Horowitz D, Hutchinson P, Kumar M, McNett M, Miller C, Naidech A, Oddo M, Olson D, O’Phelan K, Provencio JJ, Puppo C, Riker R, Robertson C, Schmidt M, Taccone F, Neurocritical Care S, European Society of Intensive Care M, (2014) Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care : a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med 40: 1189–1209 [DOI] [PubMed] [Google Scholar]
- 44.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, Nguyen S, Wusthoff CJ, Clancy RR, (2011) The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol 28: 611–617 [DOI] [PubMed] [Google Scholar]
- 45.Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, Gerard EE, Hahn CD, Husain AM, Kaplan PW, LaRoche SM, Nuwer MR, Quigg M, Riviello JJ, Schmitt SE, Simmons LA, Tsuchida TN, Hirsch LJ, (2015) Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol 32: 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payne ET, Zhao XY, Frndova H, McBain K, Sharma R, Hutchison JS, Hahn CD, (2014) Seizure burden is independently associated with short term outcome in critically ill children. Brain 137: 1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kharoshankaya L, Stevenson NJ, Livingstone V, Murray DM, Murphy BP, Ahearne CE, Boylan GB, (2016) Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Developmental medicine and child neurology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivasakumar P, Zempel J, Trivedi S, Wallendorf M, Rao R, Smith B, Inder T, Mathur AM, (2015) Treating EEG Seizures in Hypoxic Ischemic Encephalopathy: A Randomized Controlled Trial. Pediatrics 136: e1302–1309 [DOI] [PubMed] [Google Scholar]
- 49.Hunt RW, Liley HG, Wagh D, Schembri R, Lee KJ, Shearman AD, Francis-Pester S, deWaal K, Cheong JYL, Olischar M, Badawi N, Wong FY, Osborn DA, Rajadurai VS, Dargaville PA, Headley B, Wright I, Colditz PB, Newborn Electrographic Seizure Trial I, (2021) Effect of Treatment of Clinical Seizures vs Electrographic Seizures in Full-Term and Near-Term Neonates: A Randomized Clinical Trial. JAMA Netw Open 4: e2139604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bashir RA, Espinoza L, Vayalthrikkovil S, Buchhalter J, Irvine L, Bello-Espinosa L, Mohammad K, (2016) Implementation of a Neurocritical Care Program: Improved Seizure Detection and Decreased Antiseizure Medication at Discharge in Neonates With Hypoxic-Ischemic Encephalopathy. Pediatric Neurology 64: 38–43 [DOI] [PubMed] [Google Scholar]
- 51.Wusthoff CJ, Sundaram V, Abend NS, Massey SL, Lemmon ME, Thomas C, McCulloch CE, Chang T, Soul JS, Chu CJ, Rogers EE, Bonifacio SL, Cilio MR, Glass HC, Shellhaas RA, (2021) Seizure Control in Neonates Undergoing Screening vs Confirmatory EEG Monitoring. Neurology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abend NS, Chapman KE, Gallentine WB, Goldstein J, Hyslop AE, Loddenkemper T, Nash KB, Riviello JJ Jr., Hahn CD, Pediatric Critical Care EEGG, the Critical Care EEGMRC, (2013) Electroencephalographic monitoring in the pediatric intensive care unit. Curr Neurol Neurosci Rep 13: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaleski KL, Kussman BD, (2020) Near-Infrared Spectroscopy in Pediatric Congenital Heart Disease. J Cardiothorac Vasc Anesth 34: 489–500 [DOI] [PubMed] [Google Scholar]
- 54.Spilka JM, O’Halloran CP, Marino BS, Brady KM, (2021) Perspective on Cerebral Autoregulation Monitoring in Neonatal Cardiac Surgery Requiring Cardiopulmonary Bypass. Front Neurol 12: 740185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brady KM, Shaffner DH, Lee JK, Easley RB, Smielewski P, Czosnyka M, Jallo GI, Guerguerian AM, (2009) Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics 124: e1205–1212 [DOI] [PubMed] [Google Scholar]
- 56.Kirschen MP MT, Beaulieu F, Burnett R, Shaik M, Morgan RW, Baker W, Ko T, Balu R, Agarwal K, Lourie K, Sutton R, Kilbaugh T, Diaz-Arrastia R, Berg R, Topjian A., (2021) Deviations from NIRS-derived optimal blood pressure are associated with worse outcomes after pediatric cardiac arrest. Resuscitation 168: 110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien NF, Reuter-Rice K, Wainwright MS, Kaplan SL, Appavu B, Erklauer JC, Ghosh S, Kirschen M, Kozak B, Lidsky K, Lovett ME, Mehollin-Ray AR, Miles DK, Press CA, Simon DW, Tasker RC, LaRovere KL, (2021) Practice Recommendations for Transcranial Doppler Ultrasonography in Critically Ill Children in the Pediatric Intensive Care Unit: A Multidisciplinary Expert Consensus Statement. J Pediatr Intensive Care 10: 133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abecasis F, Dias C, Zakrzewska A, Oliveira V, Czosnyka M, (2021) Monitoring cerebrovascular reactivity in pediatric traumatic brain injury: comparison of three methods. Childs Nerv Syst 37: 3057–3065 [DOI] [PubMed] [Google Scholar]
- 59.Moftakhar P, Cooke DL, Fullerton HJ, Ko NU, Amans MR, Narvid JA, Dowd CF, Higashida RT, Halbach VV, Hetts SW, (2015) Extent of collateralization predicting symptomatic cerebral vasospasm among pediatric patients: correlations among angiography, transcranial Doppler ultrasonography, and clinical findings. J Neurosurg Pediatr 15: 282–290 [DOI] [PubMed] [Google Scholar]
- 60.Couret D, Boumaza D, Grisotto C, Triglia T, Pellegrini L, Ocquidant P, Bruder NJ, Velly LJ, (2016) Reliability of standard pupillometry practice in neurocritical care: an observational, double-blinded study. Crit Care 20: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boev AN, Fountas KN, Karampelas I, Boev C, Machinis TG, Feltes C, Okosun I, Dimopoulos V, Troup C, (2005) Quantitative pupillometry: normative data in healthy pediatric volunteers. J Neurosurg 103: 496–500 [DOI] [PubMed] [Google Scholar]
- 62.Freeman AD, McCracken CE, Stockwell JA, (2020) Automated Pupillary Measurements Inversely Correlate With Increased Intracranial Pressure in Pediatric Patients With Acute Brain Injury or Encephalopathy. Pediatr Crit Care Med 21: 753–759 [DOI] [PubMed] [Google Scholar]
- 63.Tosi F, Gatto A, Capossela L, Ferretti S, Mancino A, Curatola A, Chiaretti A, Pulitano S, (2021) Role of the pupillometer in the assessment of pain in the sedation of pediatric patients. Eur Rev Med Pharmacol Sci 25: 6349–6355 [DOI] [PubMed] [Google Scholar]
- 64.Opic P, Ruegg S, Marsch S, Gut SS, Sutter R, (2021) Automated Quantitative Pupillometry in the Critically Ill: A Systematic Review of the Literature. Neurology 97: e629–e642 [DOI] [PubMed] [Google Scholar]
- 65.Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TA, Parkinson C, Chung SE, Jennings JM, Jamrogowicz JJ, Larson AC, Lehmann CU, Jackson E, Brady KM, Koehler RC, Lee JK, (2013) Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res 74: 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhargava V, Tawfik D, Tan YJ, Dunbar T, Haileselassie B, Su E, (2020) Ultrasonographic Optic Nerve Sheath Diameter Measurement to Detect Intracranial Hypertension in Children With Neurological Injury: A Systematic Review. Pediatr Crit Care Med 21: e858–e868 [DOI] [PubMed] [Google Scholar]
- 67.Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, Davis-O’Reilly C, Hart EL, Bell MJ, Bratton SL, Grant GA, Kissoon N, Reuter-Rice KE, Vavilala MS, Wainwright MS, (2019) Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Pediatr Crit Care Med 20: 280–289 [DOI] [PubMed] [Google Scholar]
- 68.Robba CGF, Rebora P, (2021) Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): an international, prospective observational cohort study. . Lancet Neurology 20: 548–558 [DOI] [PubMed] [Google Scholar]
- 69.Guiza F, Depreitere B, Piper I, Citerio G, Chambers I, Jones PA, Lo TY, Enblad P, Nillson P, Feyen B, Jorens P, Maas A, Schuhmann MU, Donald R, Moss L, Van den Berghe G, Meyfroidt G, (2015) Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med 41: 1067–1076 [DOI] [PubMed] [Google Scholar]
- 70.Freeman SS UY, Armstead WM, FIsk DM, Vavilala MS, (2008) Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brian injury. Anesthesiology 108: 588–595 [DOI] [PubMed] [Google Scholar]
- 71.Vavilala MS, King MA, Yang JT, Erickson SL, Mills B, Grant RM, Blayney C, Qiu Q, Chesnut RM, Jaffe KM, Weiner BJ, Johnston BD, (2019) The Pediatric Guideline Adherence and Outcomes (PEGASUS) programme in severe traumatic brain injury: a single-centre hybrid implementation and effectiveness study. Lancet Child Adolesc Health 3: 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimony N, Martinez-Sosa M, Osburn B, Jallo GI, (2021) Non-traumatic pediatric intracranial hypertension: key points for different etiologies, diagnosis, and treatment. Acta Neurol Belg 121: 823–836 [DOI] [PubMed] [Google Scholar]
- 73.Johansson Kostenniemi U, Karlsson L, Silfverdal SA, Mehle C, (2020) MeningiSSS: A New Predictive Score to Support Decision on Invasive Procedures to Monitor or Manage the Intracerebral Pressure in Children with Bacterial Meningitis. Neurocrit Care 32: 586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dow BL, Kenardy JA, Le Brocque RM, Long DA, (2012) The utility of the Children’s Revised Impact of Event Scale in screening for concurrent PTSD following admission to intensive care. J Trauma Stress 25: 602–605 [DOI] [PubMed] [Google Scholar]
- 75.Murphy LK, Palermo TM, Meert KL, Reeder R, Dean JM, Banks R, Berg RA, Carcillo JA, Chima R, McGalliard J, Haaland W, Holubkov R, Mourani PM, Pollack MM, Sapru A, Sorenson S, Varni JW, Zimmerman J, (2020) Longitudinal Trajectories of Caregiver Distress and Family Functioning After Community-Acquired Pediatric Septic Shock. Pediatr Crit Care Med 21: 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Zellem L, Utens EM, Legerstee JS, Cransberg K, Hulst JM, Tibboel D, Buysse C, (2015) Cardiac Arrest in Children: Long-Term Health Status and Health-Related Quality of Life. Pediatr Crit Care Med 16: 693–702 [DOI] [PubMed] [Google Scholar]
- 77.Aspesberro F, Mangione-Smith R, Zimmerman JJ, (2015) Health-related quality of life following pediatric critical illness. Intensive Care Med 41: 1235–1246 [DOI] [PubMed] [Google Scholar]
- 78.Verstraete S, Van den Berghe G, Vanhorebeek I, (2018) What’s new in the long-term neurodevelopmental outcome of critically ill children. Intensive Care Med 44: 649–651 [DOI] [PubMed] [Google Scholar]
- 79.Kachmar AG, Irving SY, Connolly CA, Curley MAQ, (2018) A Systematic Review of Risk Factors Associated With Cognitive Impairment After Pediatric Critical Illness*. Pediatr Crit Care Med: e164–e171 [DOI] [PubMed] [Google Scholar]
- 80.Verstraete S, Verbruggen SC, Hordijk JA, Vanhorebeek I, Dulfer K, Guiza F, van Puffelen E, Jacobs A, Leys S, Durt A, Van Cleemput H, Eveleens RD, Garcia Guerra G, Wouters PJ, Joosten KF, Van den Berghe G, (2019) Long-term developmental effects of withholding parenteral nutrition for 1 week in the paediatric intensive care unit: a 2-year follow-up of the PEPaNIC international, randomised, controlled trial. Lancet Respir Med 7: 141–153 [DOI] [PubMed] [Google Scholar]
- 81.Watson RS, Choong K, Colville G, Crow S, Dervan LA, Hopkins RO, Knoester H, Pollack MM, Rennick J, Curley MAQ, (2018) Life after Critical Illness in Children-Toward an Understanding of Pediatric Post-intensive Care Syndrome. J Pediatr 198: 16–24 [DOI] [PubMed] [Google Scholar]
- 82.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH Jr., Li J, Smith SE, Bellinger DC, Mahle WT, American Heart Association Congenital Heart Defects Committee CoCDitYCoCN, Stroke C, (2012) Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 126: 1143–1172 [DOI] [PubMed] [Google Scholar]
- 83.Fink EL, Jarvis JM, Maddux AB, Pinto N, Galyean P, Olson LM, Zickmund S, Ringwood M, Sorenson S, Dean JM, Carcillo JA, Berg RA, Zuppa A, Pollack MM, Meert KL, Hall MW, Sapru A, McQuillen PS, Mourani PM, Watson RS, Pediatric Acute Lung I, Sepsis Investigators Long-term Outcomes Subgroup Investigators a, Eunice Kennedy Shriver National Institute of Child H, Human Development Collaborative Pediatric Critical Care Research N, (2020) Development of a core outcome set for pediatric critical care outcomes research. Contemp Clin Trials 91: 105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams CN, Hartman ME, Guilliams KP, Guerriero RM, Piantino JA, Bosworth CC, Leonard SS, Bradbury K, Wagner A, Hall TA, (2019) Postintensive Care Syndrome in Pediatric Critical Care Survivors: Therapeutic Options to Improve Outcomes After Acquired Brain Injury. Curr Treat Options Neurol 21: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]


