Abstract
Background
Familial gigantiform cementoma (FGC) is a rare benign fibrocemento-osseous lesion of the jaw characterized by well-circumscribed, extensive, mixed radiolucent-radiopaque masses in the mandible and the maxilla that can cause severe facial deformity. This condition is extremely rare with less than 40 cases reported in the literature.
Purpose
The purpose of the paper is to highlight the importance of virtual surgical planning and patient-specific implant in the treatment of a complex lesion and reconstruction of the facial skeleton. The clinical presentations, and diagnostic challenges encountered when managing the lesion have been discussed in this article with emphasis on the treatment plan.
Method/Surgical plan
The sequence of treatment planned was resection of the lesion and immediate reconstruction with a patient-specific implant to improve the patient’s quality of life. The management of FGC was a challenging one keeping in mind the rapid expansion of the lesion, widespread involvement of the jaws, and needs of the pediatric patient.
Conclusion
Virtual surgical planning (VSP) along with 3D printed implant was instrumental in reconstructing the facial form of the child where the maxilla was completely resected and rehabilitation provided support to the vital structures of the face.
Keywords: Familial gigantiform cementoma, Virtual surgical planning, Patient-specific implant
Introduction
Cemento-ossifying fibromas are benign fibro-osseous lesions that are caused by the deposition of fibrous tissue and cementum in the bone. Familial gigantiform cementoma is a rare benign subtype of cemento-osseous lesion of the jaws that can cause appreciable facial deformity and malposition of teeth affecting both the function and appearance of the face. It has an autosomal dominant mode of inheritance and is exceedingly rare with only 40 cases reported in the literature [1].
The objective of this case report is to present an unusual case of familial gigantiform cementoma manifesting with a gross expansion of the entire midface involving bilateral maxilla, zygoma and orbital floor completely obliterating the oral cavity in a 10-year-old male child and treated by a life-saving challenging resection of the entire midface and immediate reconstruction with 3D printed patient-specific titanium implant (PSI) using virtual surgical planning(VSP) technology.
Case Report
A 10-year-old male patient presented with complaints of massive swelling over both sides of the face which was increasing in size over the past 1 year which made it difficult for the patient to eat and breathe. There was a previous history of similar huge swelling in the mandible, for which resection of the mandible preserving the right condylar process was done 2 years ago and reconstructed with a titanium reconstruction plate. Family history was significant with similar swellings in 4 other family members.
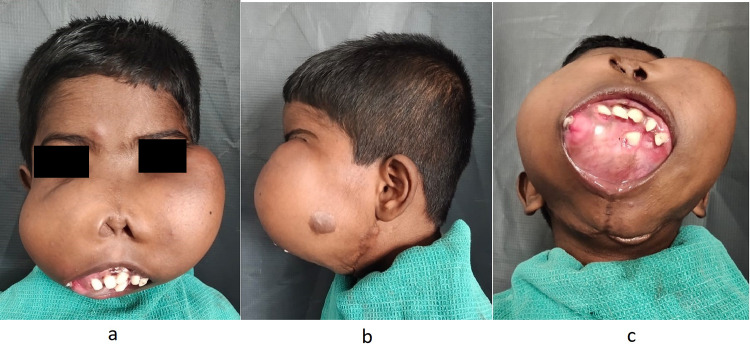
On extraoral examination, expansile swelling over the maxilla of size 15*10 cm was present obliterating the nasolabial fold on both sides extending till the ear lobes bilaterally and frontozygomatic suture superiorly, and the swelling was firm in consistency, non-tender and skin over swelling was stretched. The huge swelling made it difficult for the patient to breathe, eat or see normally (Fig. 1a, b).
Fig. 1.
a Extraoral view of the patient showing swelling over the bilateral maxilla region. b Profile view of the expansile swelling left side. c Expansile lesion involving hard palate, tumor mass displacing upper and lower lips outward with an inability to close the mouth. Multiple teeth were displaced and embedded over the mass
On intra-oral examination expansile lesion involved the hard palate, tumor mass displacing upper and lower lips outward with an inability to close the mouth, entire oral cavity was obliterated by the tumor. Multiple teeth were displaced and embedded over the mass. (Fig. 1c).
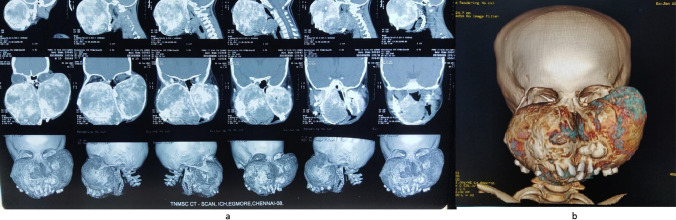
CT Facial bones revealed a bilateral expansile lesion with involvement of bilateral maxilla, zygoma, infraorbital rim, the floor of the orbit, frontozygomatic region, nasal bones, compression of the floor of both orbits and roof of oral cavity causing displacement of tongue posteriorly. (Fig. 2a, b). CT brain revealed expansile bimaxillary lesion, mild erosion of the Right Temporal bone (squamous part), medial wall of the orbit, and right ethmoid air cells. Posteriorly diffuse cortical thinning and bulging into the naso–oropharynx on the right side. Bilateral Pterygoid plate erosion was noted. An ophthalmology opinion was obtained, and vision was normal except for mechanical restrictions in the left eye due to the expanding tumor.
Fig. 2.
a CT facial bones with coronal view of the swelling extension up to left frontozygomatic region. b CT facial bones 3D view of the tumor mass with multiple teeth embedded within
The diagnosis, treatment plan and management of this life-threatening lesion were huge challenge. The goal was to restore the form and function of the face keeping in mind the growing facial skeleton of the young patient. A 3D titanium prosthesis was fabricated which was custom-made for the patient, it was intended to give a facial form after the removal of the lesion.
Surgical Plan
Preoperatively, a 3D diagnostic model was planned along with the fabrication of the prosthesis. The patient was planned for feeding gastrostomy and elective tracheostomy 2 days before the surgery. Intraoperatively, bilateral carotid control was planned. Resection of the lesion in toto and reconstruction using virtual surgical planning with patient-specific customized titanium implant was planned. Post-operatively, the chances of prolonged ventilator support were informed to the patient and high-risk informed consent was obtained.
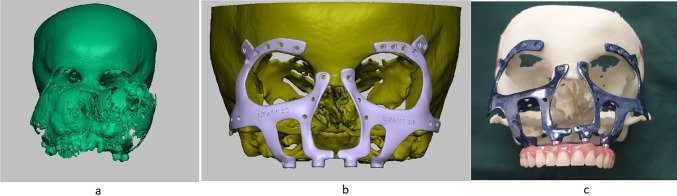
The planning process for PSI was done using the DICOM format of CT facial bones. It was carried out such that the stereolithographic model (STL) thus fabricated would have the facial bone features of a patient who is of the same age. Virtual Surgical Planning was used to estimate the portion of the tumor to be cut off, and prosthesis designing was done over the estimated remaining normal bone (Fig. 3a, b). A 3D stereolithographic model was fabricated which replicated the area post-tumor removal, and a custom-made titanium prosthesis was designed and fabricated over it (Fig. 3c).
Fig. 3.
a 3D stereolithographic model of the tumor. b Design of patient-specific implant (PSI) designed after virtual surgical planning. c Titanium Patient-specific custom-made implant designed for the patient after virtual surgical planning mounted on a 3D model
Surgical Procedure
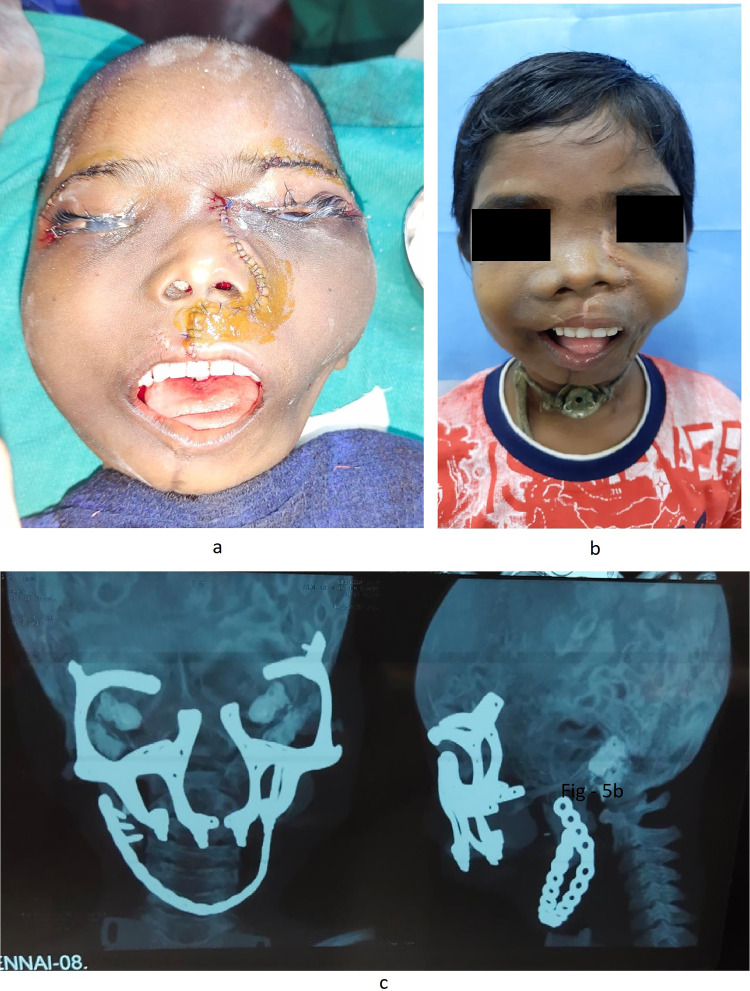
Under tracheostomy, general anesthesia was induced and maintained. Bilateral oblique incision was placed over the left submandibular triangle, layer-wise dissection was done, the external carotid artery was identified, and a vessel loop was placed. After injecting local anesthetic with adrenaline, left Weber Fergusson incision was placed with left trans-conjunctival incision and lateral canthotomy. Layer-wise dissection was done, and the lesion was identified (Fig. 4a). Osteotomy cuts were placed over the right and the left zygomatic arch. Pterygomaxillary disjunction was completed, and the lesion was resected in toto (weighing 1.5 kg) (Fig. 4b). Bilateral lateral eyebrow incisions were placed. After the periosteum was incised, the prosthesis was placed over the left and right frontal and supraorbital region with an extension over the infraorbital region and lateral nasal wall along with a denture and fixed with screws (Fig. 4c). Periosteum was closed with 3–0 vicryl, and skin closure was done using 4–0 prolene (Fig. 5a). Post-operative CT was satisfactory (Fig. 5c).
Fig. 4.
a Complete exposure of the tumor. b Resected specimen. c Titanium patient-specific implant placed over the resected area with medicated dressings over the paranasal sinus region
Fig. 5.
a Immediate post-operative picture. b post-operative picture—after 3 months. c Post-operative CT
An anterolateral thigh flap was planned and was simultaneously executed by the plastic surgery team when the debulking and tumor excision was being done by the maxillofacial surgeons’ team. But as the patient became hemodynamically unstable after tumor excision, the flap harvesting had to be discontinued and the area was sutured and monitored post-operatively.
This 3D-printed titanium prosthesis provided the necessary substructure after the complete maxilla was resected along with the tumor. The prosthesis was designed to derive support from the supraorbital region and lateral orbital rim region bilaterally to stabilize the vital structures of the face. An extension was made incorporating the orbital floor bilaterally which was covered with buccal fat pad. The obturator was attached to the prosthesis with a stud attachment and was effective in separating the oral cavity from the structures above. As this obturator was a removable one, it was effective in cleaning and irrigating the operated area which helped in wound healing.
Discussion
Gigantiform cementoma (GC) was first reported in 1930 by Norberg [1, 2] to describe a condition characterized by diffuse radiopaque masses scattered throughout the jaws. These masses frequently caused expansion. In 1953, Agazzi and Belloni described an Italian family in which several members were affected, and this was designated familial gigantiform cementoma (FGC) or familial multiple cementomas [2–4]. Most patients have an onset of growth during adolescence [5] followed by a rapid and expansive growth pattern. Expansion of the maxilla and mandible causes severe facial deformity and malocclusion due to the malposition of multiple teeth. Simple recontouring of the tumors to improve the appearance is not recommended and cosmetic shaving only results in the regrowth of the tumor at an accelerated rate [3, 6].
Finical et al. (1999) in their study of two families comprising 6 patients who presented with massive tumors concluded that even when the tumor has progressed to a massive size, resection and reconstruction can achieve a dramatic improvement in the facial appearance [4].
H.W.Wang et al.(2015) treated a 13-year-old boy with a similar lesion by mandibulectomy, followed by reconstruction with an iliac osteocutaneous free flap [7, 8]. Noffke et al. (2012) suggest that the management of the expansive FGCs entails complete surgical removal at an early stage of growth in order to limit the extent of surgery and prevent tooth displacement and loss of residual bone [9].
The diagnosis, treatment plan and management of this lesion were a huge challenge as the form and function of the face had to be restored keeping in mind the growing facial skeleton of the young patient. There were many challenges unique to the bony reconstruction of the maxillofacial skeleton, including anatomic diversity, complex movement of the mandible, saliva contamination and dental rehabilitation [10].
Computer-aided design (CAD)/computer-aided manufacturing (CAM) technology is a rapidly advancing one and has provided an avenue for the fabrication of PSIs with better precision. Maxillofacial PSIs use preoperative imaging data as input into CAD software. The designed implant is then fabricated using a CAM technique, 3D printing. This increases precision and eliminates the need for intraoperative modification of implants thus decreasing the intraoperative time [11].
Imprecise molding of off-the-shelf implants as well as the potential for graft resorption can result in suboptimal contour restoration. The recent advances in computer-aided design/computer-aided manufacturing (CAD/ CAM) have created innovative options for fabricating patient-specific implants (PSIs) with improved precision and better adaptation that improves contour outcomes.
This is a novel approach by which the contouring required to ensure the compatibility between the patient's anatomical form and the implant is eliminated. Screw positions are planned during the preoperative simulation to prevent damage to any anatomical structure. The fabrication stages include (1) Obtaining a three-dimensional model of anatomical structures from the patient’s two-dimensional scan images, (2) Simulation of the operation on the anatomical computer model, (3) Design of the PSI done according to the patient’s model and (4) Manufacturing of the implants by using proper additive production methods [12].
Virtual surgical planning (VSP) is especially a great tool in managing maxillofacial pathology for its ability to virtually visualize pathology and to provide guidance on the location of resection margins. The use of guided osteotomies is most beneficial in surgical resections of the midface and for large tumors having deformed anatomic landmarks. Challenges in midface pathology such as in our case include the difficulties in removing tumors within the maxillary sinus or nasal cavity and osteotomies that are often performed without direct visualization of the tumor. VSP allows such procedures to be performed more confidently when visual cues are absent. The proximity to vital structures of the skull base can be gauged and cutting guides designed accordingly to prevent inadvertent injury. The coupling of real-time 3D navigation and VSP further enhances these advantages to provide immediate feedback to confirm the position of guides and planned osteotomies [13].
Jacobs et al. (2017) conducted a systematic review including 315 patients who underwent three-dimensional printing-assisted operations and concluded that three-dimensional printing technology in craniomaxillofacial surgery can be classified into contour models (type I), guides (type II), splints (type III) and implants (type IV) [14].
The ideal implant material must be inexpensive, radiolucent, lightweight, durable, and biocompatible. Maxillofacial PSIs (Patient-specific implants) are commonly manufactured from metals and polymers. Titanium has been the choice metal for implant manufacturing due to its high tensile strength, lightweight and osseointegration properties. They form a protective oxide covering that resists corrosion [2]. In our case, a customized titanium patient-specific implant was made using the DICOM format of CT facial bones using virtual surgical planning [15]. The PSI was instrumental in reconstructing the facial form of the child where most of the maxilla was resected and rehabilitation provided support to the vital structures such as the eyes.
Conclusion
Reconstruction of complex maxillofacial defects is challenging especially when the tumor is extremely huge as in our case, and favorable outcomes are expected with accurate replacement of the missing or deficient tissue. Customized implants are the key to precise replacements as they have multiple benefits such as avoiding donor tissue morbidity and decreased intraoperative time when they are coupled with virtual surgical planning, and the precision is doubled bringing us a step closer to achieving the ideal implant that would bring back the premorbid form and improve the patient’s quality of life.
Acknowledgment
We would like to thank Dr. John Nesan from “Center for Technology Assisted Reconstructive Surgery (C.T.A.R.S)” Chennai and their team for the support provided for virtual surgical planning and patient-specific implant in this case.
Declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
K. Arunkumar, Email: meandarun@gmail.com
C. Prasad, Email: prasaads07@gmail.com
J. Balaji, Email: jilsba70@gmail.com
T. Rohini, Email: rohini.arasu@gmail.com
R. Supraja, Email: supraja.omfs@gmail.com
References
- 1.Prasad C, Kumar KA, Balaji J, Arulmozhi M, Jayanandhini S, Priyadharshini R. A family of familial gigantiform cementoma: clinical study. J Maxillofac Oral Surg. 2022;21(1):44–50. doi: 10.1007/s12663-021-01515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelsayed RA, Eversole LR, Singh BS, Scarbrough FE. Gigantiform cementoma: clinicopathologic presentation of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(4):438–444. doi: 10.1067/moe.2001.113108. [DOI] [PubMed] [Google Scholar]
- 3.Young SK, Markowitz NR, Sullivan S, Seale TW, Hirschi R. Familial gigantiform cementoma: classification and presentation of a large pedigree. Oral Surg Oral Med Oral Pathol. 1989;68(6):740–747. doi: 10.1016/0030-4220(89)90165-5. [DOI] [PubMed] [Google Scholar]
- 4.Finical SJ, Kane WJ, Clay RP, Bite U. Familial gigantiform cementoma. Plast Reconstr Surg. 1999;103(3):949–954. doi: 10.1097/00006534-199903000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Rossbach HC, Letson D, Lacson A, Ruas E, Salazar P. Familial gigantiform cementoma with brittle bone disease, pathologic fractures and osteosarcoma: a possible explanation of an ancient mystery. Pediatr Blood Cancer. 2005;44:390–396. doi: 10.1002/pbc.20253. [DOI] [PubMed] [Google Scholar]
- 6.Yadav S, Bal M, Qureshi S. Familial gigantiform cementoma with calcium steal phenomenon and social stigma: a case report with review of literature. J Maxillofac Oral Surg. 2021 doi: 10.1007/s12663-021-01521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HW, Yu M, Qin XJ, Zhang CP. Familial gigantiform cementoma: distinctive clinical features of a large Chinese pedigree. Br J Oral Maxillofac Surg. 2015;53(1):83–85. doi: 10.1016/j.bjoms.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Wang H-W, Ma C-Y, Qin X-J, Zhang C-P. Management strategy in patient with familial gigantiform cementoma. Medicine. 2017 doi: 10.1097/md.0000000000009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noffke C, Ngwenya S, Nzima N, Raubenheimer E, Rakgwale N. Gigantiform cementoma in a child. Dentomaxillofac Radiol. 2012;41(3):264–266. doi: 10.1259/dmfr/13435626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang MF, Alfi D, Alfi J, Huang AT. The use of patient-specific implants in oral and maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2019;31(4):593–600. doi: 10.1016/j.coms.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Owusu JA, Boahene K. Update of patient-specific maxillofacial implant. Curr Opin Otolaryngol Head Neck Surg. 2015;23(4):261–264. doi: 10.1097/MOO.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 12.Yagiz A, Dogru SC, Üzel M, Kocaelli H, Arslan YZ, Cansiz E (2021) Design of patient-specific maxillofacial implants and guides. In: Sharma NR, Subburaj K, Sandhu K, Sharma V (eds) Applications of 3D printing in biomedical engineering. Springer, Singapore. 10.1007/978-981-33-6888-0_5
- 13.Hua J, Aziz S, Shum JW. Virtual surgical planning in oral and maxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2019;31(4):519–530. doi: 10.1016/j.coms.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs CA, Lin AY. A new classification of three-dimensional printing technologies: sya stematic review of three-dimensional printing for patient-specific craniomaxillofacial surgery. Plast Reconstr Surg. 2017;139(5):1211–1220. doi: 10.1097/PRS.0000000000003232. [DOI] [PubMed] [Google Scholar]
- 15.Alasseri N, Alasraj A. Patient-specific implants for maxillofacial defects: challenges and solutions. Maxillofac Plast Reconstr Surg. 2020;42:15. doi: 10.1186/s40902-020-00262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]