Figure 5.
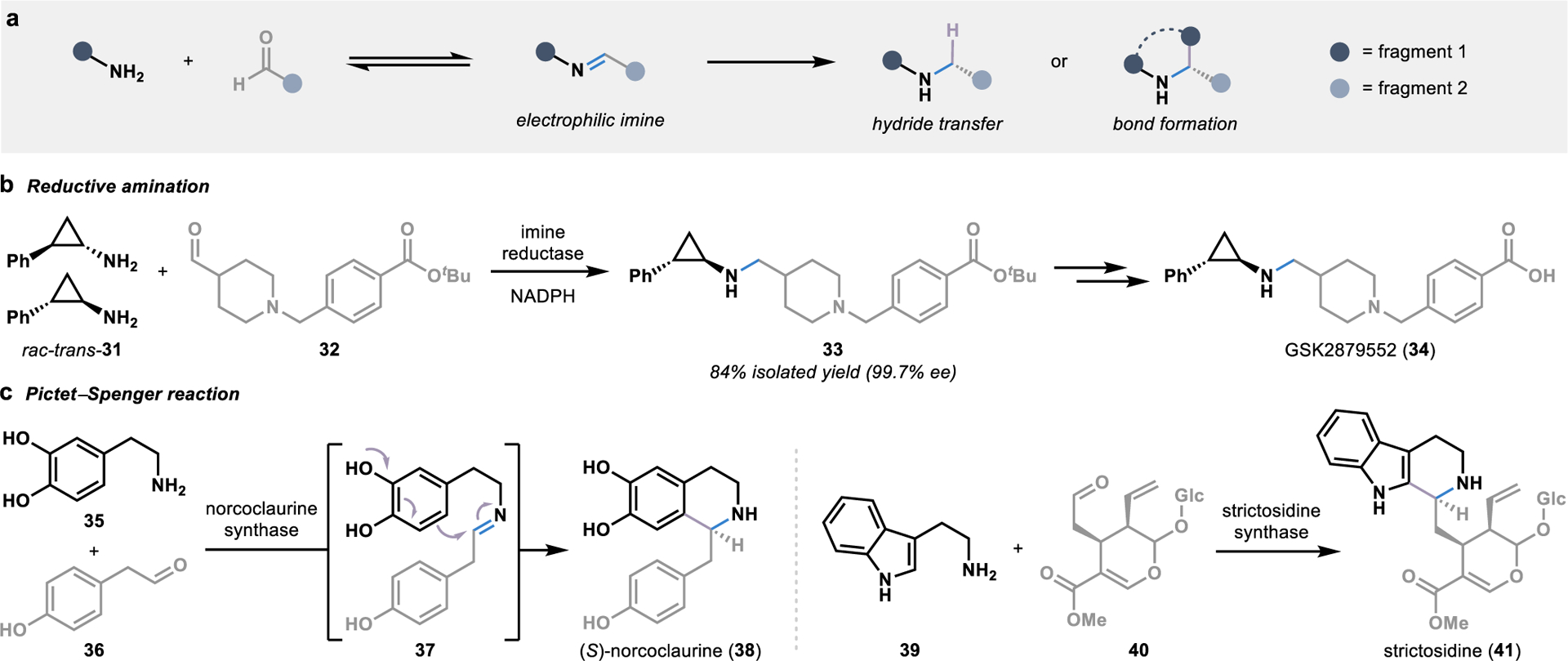
Biocatalytic strategies for convergent amination reactions. (a) The condensation of fragments bearing an amine and an aldehyde forms electrophilic imine intermediates poised for stereoselective functionalization by an enzyme. (b) Imine reductases utilize NADPH to stereoselectively delivery a hydride to an imine intermediate in the biocatalytic synthesis of chiral amines. (c) Pictet-Spenglerases harness imine intermediates in the biosynthesis of alkaloid metabolites, allowing for the intramolecular formation of a C-C bond through an electrophilic aromatic substitution reaction.
