Figure 7.
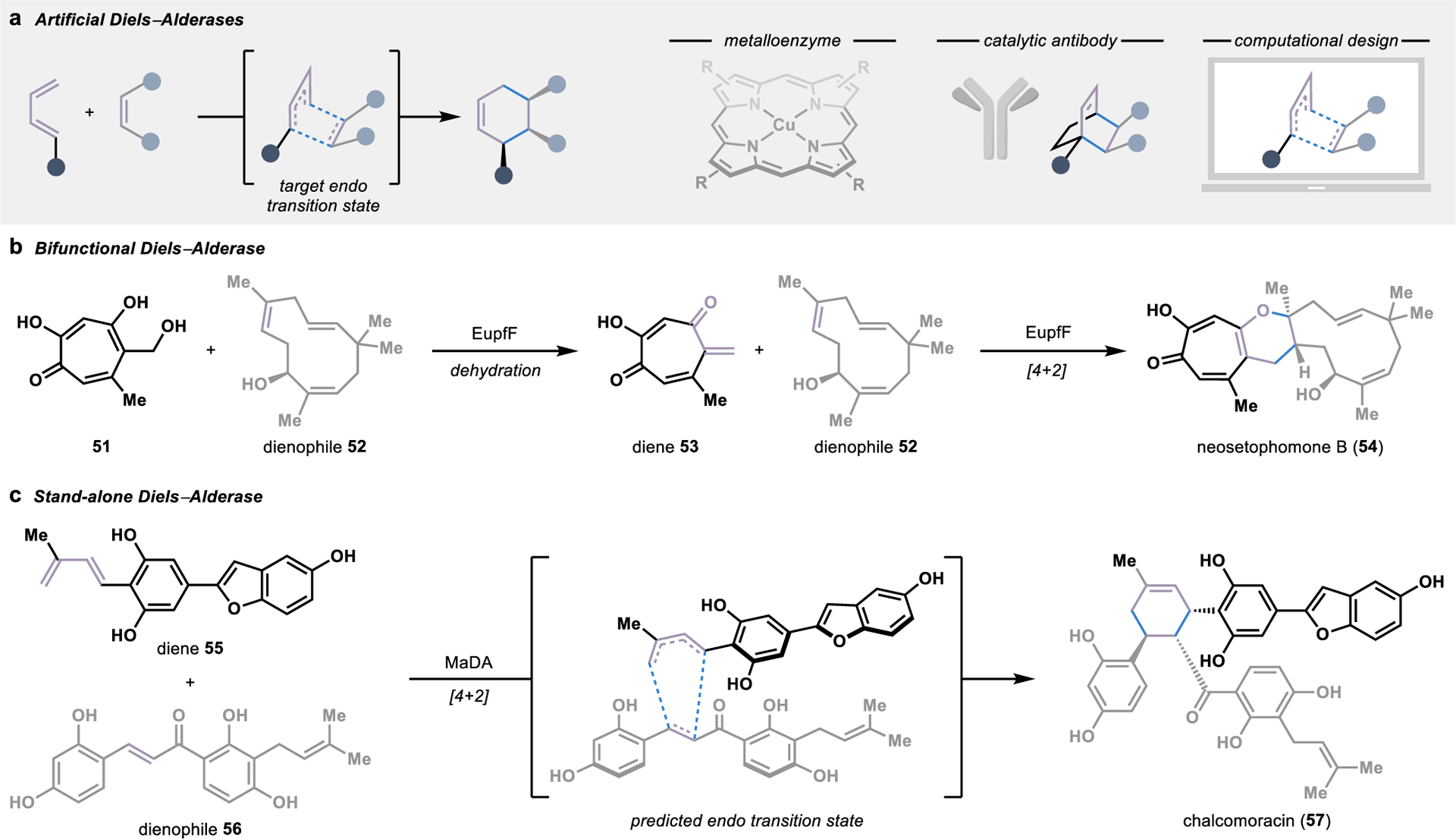
Biocatalytic strategies for intermolecular Diels–Alder reactions. (a) Artificial Diels–Alderases can be engineered by anchoring a metal cofactors into an enzyme cavity, training catalytic antibodies against a target transition state mimic, or computation design. (b) A bifunctional Diels–Alderase in the biosynthesis of neosetophomone B (54) catalyzes both the dehydration to form diene 53 and a [4+2] cycloaddition. (c) A stand-alone natural Diels–Alderase that catalyzes a concerted, intermolecular [4+2] cycloaddition was first discovered in the biosynthesis of chalcomoracin (57).
