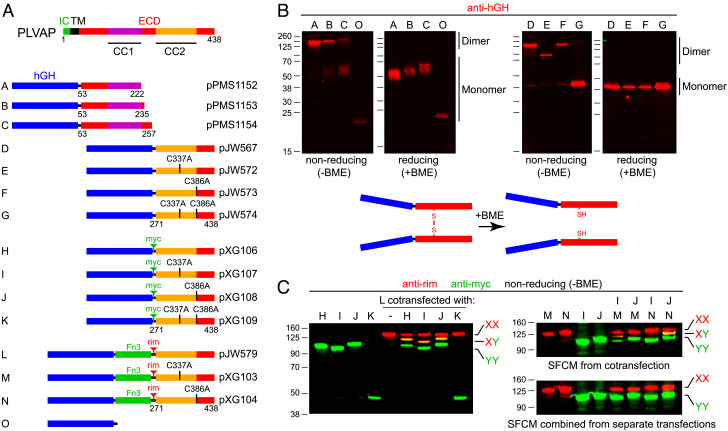
Fig. 3.
Biochemical evidence that PLVAP ECD fragments form parallel homodimers linked by disulfide bonds between corresponding cysteines. (A) Map of full-length PLVAP (Top) and secreted human growth hormone (hGH; blue rectangle) fusions with the indicated ECD fragments (Below). The CC2 region of PLVAP has two cysteines at positions 337 and 386, and various derivatives have either one or both mutated to alanine, as indicated for the fusion proteins labeled D to N. The tenth fibronectin type 3 (Fn3) domain from human fibronectin (amino acids 1,538 to 1,630) is shown as a green rectangle in fusion proteins L, M, and N. (B) SDS-PAGE and anti-hGH immunoblots of serum-free conditioned medium from transiently transfected HEK/293T cells showing the electrophoretic mobilities of the indicated fusion proteins under either reducing [+beta-mercaptoethanol (BME)] or nonreducing [-BME] conditions. Fusion proteins D, E, and F have identical lengths but exhibit distinct electrophoretic mobilities under nonreducing conditions based on the location of the disulfide bonds. Fusion protein G, which lacks interchain disulfide bonds, migrates as a monomer after heating in SDS in the absence of BME. D, dimer. M, monomer. (C) SDS-PAGE and anti-epitope tag immunoblots of serum-free conditioned medium (SFCM) showing that coexpression and cosecretion of pairs of PLVAP ECD fusion proteins with different electrophoretic mobilities and different epitope tags lead to both homo- and heterodimer formation. The dimers are stable to heating in SDS in the absence of BME if there is at least one interchain disulfide bond. Heterodimers do not form if SFCM containing the individual homodimeric fusion proteins are mixed together prior to gel electrophoresis. Immunoblotting was performed with mouse anti-rim mAb and rabbit anti-myc, and the primary antibodies were visualized with fluorescent secondary antibodies.