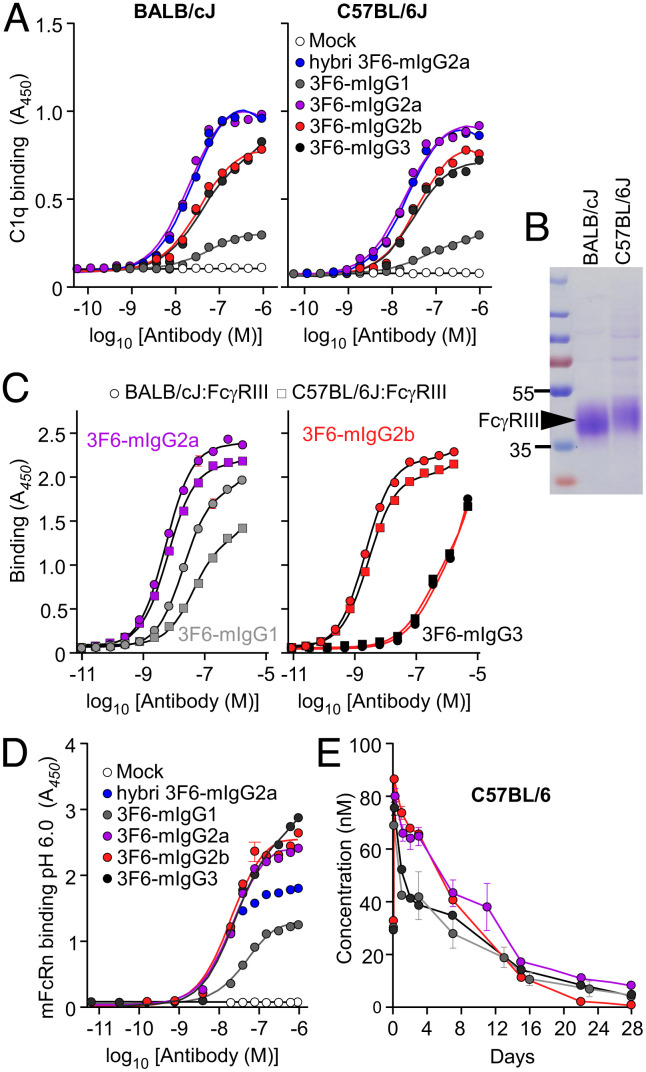
Fig. 4.
Assessing the impact of C1q, FcγRIII and FcRn polymorphisms for interaction with antibodies. (A) Interactions between 3F6-mIgGs and C1q (n = 3 assays) were assessed using ELISA plates coated with test antibodies or mock (PBS) and incubated with BALB/cJ (Left) and C57BL/6J (Right) mouse sera. Bound C1q was detected by HRP-conjugated anti-mouse C1q. (B) Coomassie-stained gel of the extracellular domain of C57BL/6J and BALB/cJ FcγRIII purified from transfected HEK-293F cells. (C) Interactions between 3F6-mIgGs and the extracellular domain of BALB/cJ and C57BL/6J FcγRIII (n = 3 assays) were assessed using ELISA plates coated with purified FcγRIII. Bound antibodies were detected with HRP-conjugated anti-mIgG. (D) Interactions between 3F6-mIgGs and mFcRn (n = 3 assays) were measured at pH 6.0 using ELISA plates coated with test antibodies or mock (PBS). Bound biotinylated FcRn was detected with HRP-conjugated streptavidin. (E) Serum concentration over time of four 3F6-mIgGs in C57BL/6J mice (n = 5 mice per group). Absorbances (A, C, and D) were recorded at 450 nm (A450). Data (A, C, D, and E) are presented as mean ± SEM and are representative of at least two independent experiments.