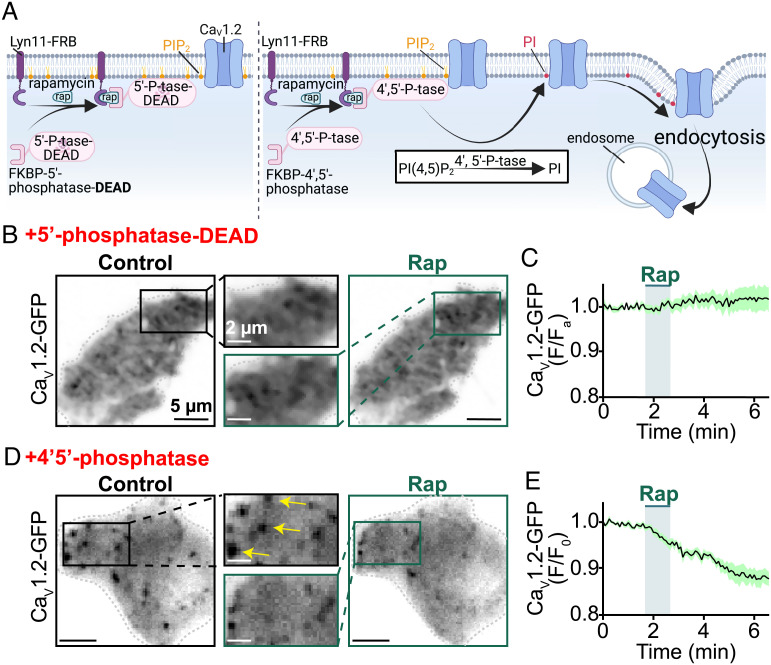
Fig. 2.
Receptor-independent PIP2 depletion via chemical translocation of a lipid phosphatase destabilizes plasma membrane CaV1.2 expression. (A) Illustration of the FKBP–FRB rapamycin-dependent dimerization system used to irreversibly recruit an enzymatically dead (control; Left) or an active (Right) lipid phosphatase to the PM. The active enzyme depletes PIP2 by metabolizing it into PI. The cartoon graphically illustrates the testable prediction that this would destabilize PM expression of CaV1.2 and trigger their endocytosis. (B) TIRF images and (C) average CaV1.2-GFP (F/F0) time-course showing PM CaV1.2-GFP expression in transfected tsA201 cells before and after rapamycin (1 µM) induced recruitment of enzymatically dead 5′-lipid-phosphatase (n = 7). (D) TIRF images and (E) average CaV1.2-GFP (F/F0) time-course showing modulation of PM CaV1.2-GFP expression in transfected tsA201 cells before and after rapamycin-induced recruitment of active 4′,5′-phosphatase (pseudojanin-FKBP; n = 6).