Abstract
Objective
To assess outcomes in giant cell arteritis (GCA) patients during and after long-term tocilizumab (TCZ) treatment.
Methods
Retrospective analysis of GCA patients treated with TCZ at a single centre (2010–2022). Time to relapse and annualised relapse rate during and after TCZ treatment, prednisone use, and safety were assessed. Relapse was defined as reappearance of any GCA clinical manifestation that required treatment intensification, regardless of C reactive protein levels and erythrocyte sedimentation rate.
Results
Sixty-five GCA patients were followed for a mean (SD) of 3.1 (1.6) years. The mean duration of the initial TCZ course was 1.9 (1.1) years. The Kaplan-Meier (KM)-estimated relapse rate at 18 months on TCZ was 15.5%. The first TCZ course was discontinued due to satisfactory remission achievement in 45 (69.2%) patients and adverse events in 6 (9.2%) patients. KM-estimated relapse rate at 18 months after TCZ discontinuation was 47.3%. Compared with patients stopping TCZ at or before 12 months of treatment, the multivariable adjusted HR (95% CI) for relapse in patients on TCZ beyond 12 months was 0.01 (0.00 to 0.28; p=0.005). Thirteen patients received >1 TCZ course. Multivariable adjusted annualised relapse rates (95% CI) in all periods on and off TCZ aggregated were 0.1 (0.1 to 0.2) and 0.4 (0.3 to 0.7), respectively (p=0.0004). Prednisone was discontinued in 76.9% of patients. During the study, 13 serious adverse events occurred in 11 (16.9%) patients.
Conclusion
Long-term TCZ treatment was associated with remission maintenance in most patients with GCA. The estimated relapse rate by 18 months after TCZ discontinuation was 47.3%.
Keywords: Giant Cell Arteritis, Biological Therapy, Systemic vasculitis
WHAT IS ALREADY KNOWN ON THIS TOPIC
The optimal duration of treatment for patients with giant cell arteritis (GCA) has not been defined. The rate of disease relapse after 1 year of treatment with tocilizumab (TCZ) is approximately 60%. Disease outcomes following longer TCZ treatment courses are currently unknown.
WHAT THIS STUDY ADDS
These results showed that after receiving TCZ for a mean period of 1.6 years, the estimated relapse rate by 18 months after TCZ discontinuation was nearly 50%. The incidence of adverse events exclusively related to TCZ declined over time.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These results could aid clinical decision making when discussing with GCA patients the risks and benefits of continuation vs discontinuation of TCZ after 12 months of treatment.
Introduction
Disease relapse and glucocorticoid-related toxicity are among the most important problems associated with giant cell arteritis (GCA). Approximately 65% of patients relapse when receiving glucocorticoids only,1 2 and most develop glucocorticoid-related adverse events.3 Randomised controlled trials evaluating the efficacy of methotrexate for prevention of GCA relapse have shown conflicting results.4–6 However, observational studies and a meta-analysis of three clinical trials suggest that methotrexate may offer modest remission maintenance and glucocorticoid-sparing benefits.7 8 Abatacept, secukinumab and mavrilimumab have been shown to decrease the risk of relapse in phase II studies, which require confirmation in phase III.9–11
To date, tocilizumab (TCZ) is the only medication with confirmed efficacy in terms of remission maintenance, glucocorticoid sparing and health-related quality of life when used for 1 year in patients with new-onset and relapsing GCA.12 13 Nevertheless, the optimal duration of TCZ treatment for GCA is currently unclear. Between 15% and 30% of patients relapse within the first year of TCZ treatment.12–15 In addition, the observational long-term extension of the Phase III GiACTA trial12 showed that 58% of patients who stopped weekly TCZ injections while in remission after 12 months of treatment relapsed within 2 years.16 The outcomes in patients receiving TCZ for longer periods of time and in particular the rate of relapse after discontinuation of long-term TCZ treatment have been scarcely investigated.17
The primary objective of this study was to evaluate the effectiveness and safety of long-term TCZ use in patients with GCA, to estimate the relapse rate after TCZ discontinuation and to understand the impact of TCZ treatment duration on the risk of relapse.
Methods
Study design and patient population
A retrospective analysis was conducted using electronic medical records (EMRs) from the first 65 patients who had received a diagnosis of GCA and were treated with intravenous or subcutaneous TCZ for at least 9 months by rheumatologists at Massachusetts General Hospital (Boston, Massachusetts, USA) between 2010 and 2022. The sample size was chosen based on feasibility. Although a TCZ treatment duration of less than 12 months is not considered standard of care, the inclusion of patients whose initial TCZ course was slightly less than 12 months increased the sample size and allowed us to create two defined groups (ie, those receiving TCZ for 12 months or less and those receiving TCZ for more than 12 months) for the assessment of TCZ treatment duration on the risk of relapse. All patients met the 1990 American College of Rheumatology classification criteria for GCA. Patients received TCZ at the discretion of the treating rheumatologist either for newly diagnosed disease or relapsed disease despite the use of glucocorticoids (eg, prednisone). Because this was a retrospective study of real-world data generated by a group of independent rheumatologists, there were no predetermined criteria to select the dose and route of administration of TCZ or determine the duration of TCZ therapy. Factors influencing providers when making those decisions may have included patient’s preference, provider’s experience and judgement, insurance authorisation, cost and safety.
Study assessments
Efficacy and safety outcomes and patterns of prednisone use were evaluated during TCZ treatment and after TCZ discontinuation by reviewing all rheumatology notes and laboratory values available in each patient’s EMR. During the follow-up, patients were seen in the clinic at variable intervals, but mostly every 1–6 months. The duration of treatment with TCZ was defined according to the best clinical judgement of each treating rheumatologist with reasons for TCZ discontinuation including satisfactory achievement of disease control for an adequate period of time (ie, long-term remission), adverse event or treatment inefficacy. The primary outcome was the occurrence of disease relapse, defined as the reappearance of unequivocal clinical manifestations of GCA that required treatment intensification.1 12 13 Because of the known effects of interleukin-6 receptor blockade on acute phase reactant levels, C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were not considered in the decision to intensify treatment for disease relapse. Additional outcomes were time to relapse, annualised relapse rate, clinical characteristics of relapse, ability to discontinue prednisone and occurrence of adverse events and serious adverse events.
Statistical analysis
Patient baseline characteristics and efficacy and safety outcomes were summarised using descriptive statistics. Wilcoxon signed rank test was used to compare the prednisone dose at TCZ initiation and at last follow-up. The relapse-free probabilities during the initial TCZ treatment course and after discontinuation of the initial TCZ treatment course were estimated by the Kaplan-Meier method. Patients were considered at risk for relapse on TCZ treatment during TCZ use and up to 7 days after receiving the last weekly subcutaneous injection, 14 days after receiving the last every-other-week subcutaneous injection and 28 days after receiving the last monthly intravenous infusion.
To estimate the risk of relapse associated with different lengths of TCZ treatment, we divided the cohort of patients into a group discontinuing the first TCZ course before or at 12 months (shorter TCZ treatment group) and a group continuing the first TCZ course beyond 12 months (longer TCZ treatment group). We then performed Cox regression analysis of time to relapse with origin at the time of TCZ discontinuation in the shorter TCZ treatment group and at 12 months in the longer TCZ treatment group. The Cox regression model included treatment group (ie, shorter or longer TCZ treatment), sex, occurrence of flare during the initial 9–12 months of TCZ treatment and duration of time between prednisone discontinuation and the point of origin of the analysis. Starting prednisone dose (>30 or ≤30 mg/day) and disease type (new onset vs relapsing) at the beginning of the first TCZ course were additional covariates examined. Patients in the shorter TCZ treatment group were censored in case of reinitiation of TCZ for a reason other than relapse or when their follow-up ended. Patients in the longer TCZ treatment group were censored in case of TCZ discontinuation or when their follow-up ended. A multivariable adjusted HR and 95% CIs were reported.
Some patients received more than one TCZ treatment course. Annualised relapse rates were estimated from a Poisson regression model aggregating all periods on TCZ and all periods off TCZ after the first TCZ course. Rates are estimated from a Poisson regression model, with ongoing TCZ treatment (ie, on or off TCZ), age, relapse status, sex and prednisone dose at TCZ initiation as covariates and random patient effect. Multivariable adjusted annualised relapse rates and 95% CIs were reported. The relapse rate ratio was computed as the ratio of the rate of relapse on TCZ by the rate of relapse off TCZ.
Results
Baseline demographics and clinical characteristics
A total of 65 patients with GCA were followed for a mean (SD) period of 3.1 (1.6) years. Baseline patient characteristics are shown in table 1. Most were female (67.7%), and the mean (SD) age was 70.8 (9.2) years. GCA was confirmed by temporal artery biopsy in 40 (61.5%) patients and by vascular imaging in 21 (32.3%) patients. Overall, 27 (41.5%) patients started TCZ on disease diagnosis and 38 (58.5%) patients started TCZ after disease relapse. Most patients (67.7%) received TCZ 162 mg subcutaneously once a week. All patients were on prednisone at the time of TCZ initiation. Only 2 (3.1%) received methotrexate concomitantly with TCZ including one patient who previously had inadequate responses to abatacept, tofacitinib and secukinumab. Another seven patients were treated with other immunosuppressants before receiving TCZ. Those included four patients who had an inadequate response to methotrexate, two patients who had an inadequate response to ustekinumab and one patient who had an inadequate response to methotrexate and cyclophosphamide.
Table 1.
Baseline characteristics and treatments of patients with GCA
| (N=65) | |
| Age, mean (SD) | 70.8 (9.2) |
| Female sex | 44 (67.7) |
| Biopsy-proven disease | 40 (61.5) |
| Imaging-proven disease* | 21 (32.3) |
| New-onset disease at TCZ initiation† | 27 (41.5) |
| Initial TCZ treatment | |
| 4 mg/kg intravenously monthly | 2 (3.1) |
| 8 mg/kg intravenously monthly | 13 (22.0) |
| 162 mg subcutaneously every 2 weeks | 6 (9.2) |
| 162 mg subcutaneously weekly | 44 (67.7) |
| Use of other immunosuppressants for GCA | |
| Concurrent with TCZ | 2 (3.1)‡ |
| Previous to TCZ | 8 (12.3)§ |
| On prednisone at TCZ initiation | 65 (100) |
| Daily prednisone dose (mg) at TCZ initiation, mean (SD) | 33.9 (21.5) |
| Patients receiving >1 TCZ course | 13 (20.0) |
| Duration of first TCZ course (years), mean (SD) | 1.9 (1.1) |
| Total duration of TCZ treatment (years), mean (SD) | 2.2 (1.6)¶ |
Values represent number and (%) unless otherwise specified.
*Ultrasound, CT angiography, MR angiography or positron emission tomography showing arterial lesions suggestive of vasculitis.
†TCZ started within 12 weeks of GCA diagnosis without preceding disease flare.
‡Methotrexate in both cases.
§Including methotrexate, cyclophosphamide, ustekinumab, abatacept, tofacitinib and secukinumab.
¶Aggregated time on TCZ for patients who received more than one TCZ course.
GCA, giant cell arteritis; TCZ, tocilizumab.
Disease relapse during and after TCZ treatment
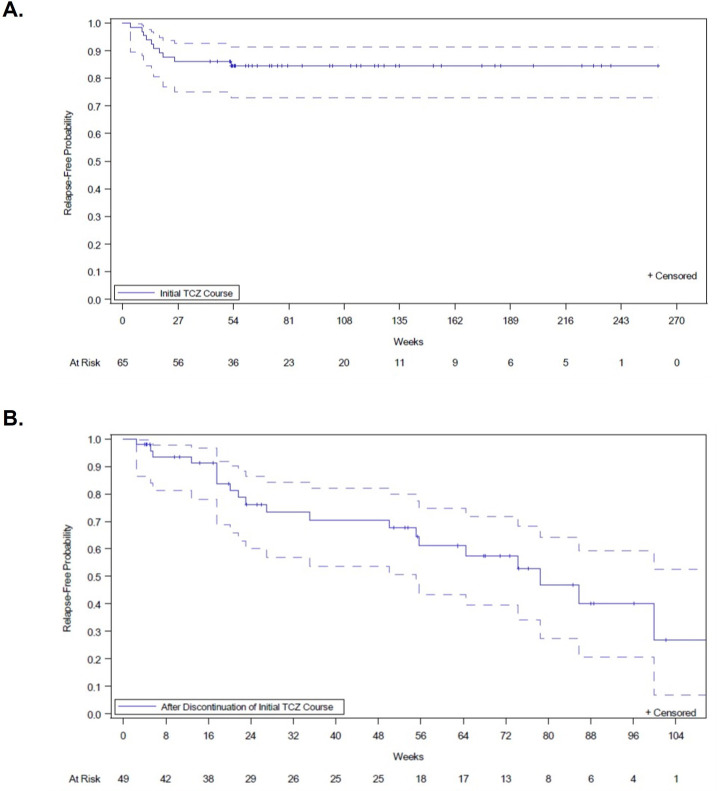
The mean (SD) duration of the first TCZ course for the entire cohort was 1.9 (1.1) years (table 1), with nearly 80% of patients having the first TCZ course lasting at least 12 months. While on the first TCZ course, 10 (15.4%) patients relapsed. The Kaplan-Meier-estimated relapse rate during the first TCZ treatment was 13.8% at 12 months and 15.5% at 18 months (figure 1). After a mean (SD) period of 1.6 (0.9) years of treatment, TCZ was discontinued due to long-term remission in 45 (69.2%) patients and following adverse events in 6 (9.2%) patients. By the time of TCZ discontinuation, 47 of these 51 patients had weaned off prednisone completely, with a mean (SD) time between prednisone discontinuation and TCZ discontinuation of 7.3 (3.6) months. After TCZ discontinuation, 49 patients had additional follow-up; of those, 20 (40.8%) subsequently relapsed. The Kaplan-Meier-estimated relapse rate after stopping the first TCZ course was 32.3% at 12 months and 47.3% at 18 months (figure 1).
Figure 1.
Time to first GCA relapse during and after the first tocilizumab course. (A) Time to first disease relapse during first TCZ course. Patients were considered at risk for relapse after TCZ initiation until completion of the first TCZ course plus 7 days in patients receiving subcutaneous weekly TCZ, 14 days in patients receiving subcutaneous every-other-week TCZ or 28 days in patients receiving monthly intravenous TCZ. (B) Time to first disease flare after the first TCZ course. Dotted lines represent 95% CIs. GCA, giant cell arteritis; TCZ, tocilizumab.
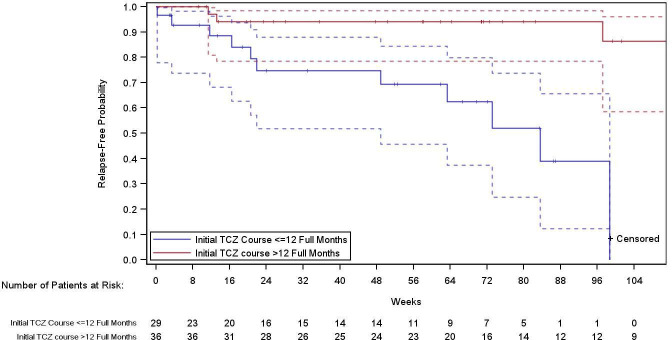
Overall, 29 patients received a first TCZ course of 9–12 months (shorter TCZ treatment group), and 36 received a first TCZ course lasting more than 12 months (longer TCZ treatment group). The multivariable adjusted HR (95% CI) for relapse in patients with longer TCZ use after 12 months of treatment relative to those with shorter TCZ use after drug discontinuation was 0.01 (0.00 to 0.28; p=0.005) (figure 2).
Figure 2.
Time to first GCA relapse according to the duration of tocilizumab treatment. Blue line represents patients on initial TCZ treatment for 9–12 months (group 1). Patients in group 1 were censored if they stopped TCZ or follow-up ended. Red line represents patients on initial TCZ treatment for more than 12 months (group 2). Patients in group 2 were censored if they restarted TCZ for a reason other than relapse or follow-up ended. Dotted lines represent 95% CIs. GCA, giant cell arteritis; TCZ, tocilizumab.
A total of 13 (20%) patients received more than one course of TCZ treatment during the follow-up period. The aggregation of all TCZ courses (median 1.7 years) and all periods off TCZ after the first TCZ treatment (median 1 year) showed that 14 patients (21.5%) relapsed while receiving TCZ and 21 (32.3%) relapsed during time off TCZ (table 2). The multivariable adjusted annualised relapse rate (95% CI) was 0.1 (0.1 to 0.2) during TCZ treatment and 0.4 (0.3 to 0.7) during time off TCZ (rate ratio, 0.3; p=0.0004) (table 2).
Table 2.
Annualised relapse rate on and off tocilizumab treatment
| On TCZ (N=65) |
Off TCZ (N=65) |
Overall (N=65) |
|
| Follow-up time, median (IQR), years | 1.7 (1.0–3.0) | 1.0 (0.4–1.6) | 2.8 (2.1–3.9) |
| Rate of flares per year* | |||
| Rate (95% CI) | 0.14 (0.09 to 0.22) | 0.42 (0.27 to 0.65) | |
| Rate ratio (95% CI)† | 0.33 (0.18 to 0.60) | ||
| P value | 0.0004 | ||
| Patients with ≥1 flare, n (%) | 14 (21.5) | 21 (32.3) | 31 (47.7) |
| 1 flare | 8 (12.3) | 16 (24.6) | 20 (30.8) |
| 2 flares | 4 (6.2) | 5 (7.7) | 6 (9.2) |
| 3 flares | 2 (3.1) | 0 | 4 (6.2) |
| ≥4 flares | 0 | 0 | 1 (1.5) |
Log of the time at risk is used as an offset variable. All follow-up time is included in the time at risk for a new event. The TCZ analysis population is defined as the participants whose initial TCZ course was ≥9 months.
* Rates are estimated from a Poisson regression model, with ongoing TCZ treatment, age, relapse status, sex, and prednisone dose at TCZ intiation as covariates, and random patient effect. For both on TCZ and after TCZ discontinuation, the time period considered is aggregated for all periods on and off TCZ, respectively, after TCZ initiation.
† Rate ratio was computed as the ratio of the rate on TCZ by the rate off TCZ.
TCZ, tocilizumab.
Characteristics of disease relapse
A total of 22 relapses occurred in 14 patients while on TCZ, compared with 26 relapses in 21 patients during time off TCZ (table 3). Relapses occurring on TCZ exhibited cranial and polymyalgia rheumatica manifestations in 59.1% and 40.9% of cases, respectively, including visual symptoms in 1 patient who developed permanent vision loss. As expected, ESR≥30 mm/hour and CRP≥10 mg/L were observed in only 4 (18.2%) and 2 (9.1%) relapses, respectively. The mean (SD) ESR and CRP values at the time of relapse on TCZ were 16.6 (21.5) mm/hour and 5.2 (10.6) mg/L, respectively (table 3).
Table 3.
Characteristics of GCA relapses occurring on and off tocilizumab treatment
| On TCZ (N=65) |
Off TCZ (N=65) |
|
| No of relapses | 22 | 26 |
| Symptoms | ||
| Symptoms of PMR | 9 (40.9) | 20 (76.9) |
| Cranial symptoms* | 13 (59.1) | 8 (30.8) |
| Visual symptoms† | 1 (4.5) | 1 (3.8) |
| Permanent vision loss | 1 (4.5) | 0 |
| On prednisone | 12 (54.5) | 2 (7.7) |
| Off prednisone | 10 (45.5) | 24 (92.3) |
| Prednisone dose at time of relapse, mg/day | ||
| Mean (SD) | 6.2 (9.8) | 0.5 (1.8) |
| ESR at time of relapse, mm/hour‡, § | ||
| Mean (SD) | 16.6 (21.5) | 31.4 (25.4) |
| ≥30 mm/hour | 4 (18.2) | 11 (42.3) |
| CRP at time of relapse, mg/L¶,** | ||
| Mean (SD) | 5.2 (10.6) | 13.3 (22.1) |
| ≥10 mg/L | 2 (9.1) | 7 (26.9) |
All values represent number (%) unless otherwise specified. The denominator for all values is the total number of relapses in each group.
*Cranial symptoms include the following: headache, jaw claudication and scalp tenderness, amaurosis fugax, blurred vision, diplopia and/or permanent vision loss.
†Visual symptoms include the following: amaurosis fugax, blurred vision, diplopia and/or permanent vision loss.
‡ESR not available in six relapses on TCZ.
§ESR not available in three relapses off TCZ.
¶CRP not available in seven relapses on TCZ.
**CRP not available in nine relapses off TCZ.
CRP, C reactive protein; ESR, erythrocyte sedimentation rate; GCA, giant cell arteritis; PMR, polymyalgia rheumatica; TCZ, tocilizumab.
Relapses occurring during time off TCZ exhibited cranial and polymyalgia rheumatica symptoms in 30.8% and 76.9% of cases, respectively, including visual manifestations in 1 patient without permanent vision loss. ESR≥30 mm/hour and CRP≥10 mg/L were observed in 11 (42.3%) and 7 (26.9%) relapses, respectively. The mean (SD) ESR and CRP values at the time of relapse off TCZ were 31.4 (25.4) mm/hour and 13.3 (22.1) mg/L, respectively (table 3).
Prednisone use
The mean (SD) daily prednisone dose was 33.9 (21.5) mg at the time of TCZ initiation and 2.6 (8.4) mg by the end of follow-up (p<0.0001 for comparison with the dose at TCZ initiation). Following TCZ initiation, the entire cohort of 65 patients required prednisone for a mean (SD) period of 10.6 (14.3) months. At last follow-up, 50 (76.9%), patients had been able to wean off prednisone.
Of the 22 flares occurring on TCZ, 12 (54.5%) developed while patients were still on prednisone (mean daily dose 6.2 mg) and 10 (45.5%) occurred after prednisone discontinuation (table 3). Of the 26 flares occurring during time off TCZ after the first TCZ course, 24 (92.3%) happened when patients were not receiving prednisone and 2 (7.7%) developed in patients receiving prednisone (mean daily dose 0.5 mg) (table 3).
Safety
A total of 48 (73.8%) patients developed at least one adverse event during 204.1 patient-years of follow-up, including 121 non-serious adverse events and 13 serious adverse events (table 4). Among the 121 non-serious adverse events, 23 (19.0%) were related or possibly related to prednisone exclusively, 48 (39.7%) were related or possibly related to TCZ exclusively and 9 (7.4%) were related or possibly related to prednisone or TCZ (table 4). Among the 13 serious adverse events, 3 (23.1%) were related or possibly related to prednisone exclusively, 4 (30.8%) were related or possibly related to TCZ exclusively and 2 (15.5%) were related or possibly related to prednisone or TCZ (table 4).
Table 4.
Safety in patients with GCA
| (N=65) | |
| Patients with ≥1 AE | 48 (73.8) |
| Total no of non-serious AEs | 121 |
| Related or possibly related to prednisone exclusively* | 23 (19.0) |
| Related or possibly related to TCZ exclusively* | 48 (39.7) |
| Related or possibly related to prednisone or TCZ* | 9 (7.4) |
| Patients with ≥1 SAE | 11 (16.9) |
| Total no of SAEs | 13 |
| Related or possibly related to prednisone exclusively† | 3 (23.1) |
| Diabetic ketoacidosis | 1 |
| Stroke | 1 |
| Vertebral fracture | 1 |
| Related or possibly related to TCZ exclusively† | 4 (30.8) |
| Bacteraemia of unclear source | 1 |
| COVID-19 | 1 |
| Diverticulitis | 1 |
| Postsurgical skin and soft-tissue infection | 1 |
| Related or possibly related to prednisone or TCZ† | 2 (15.4) |
| Pneumonia | 1 |
| Sepsis from urinary tract infection | 1 |
All values represent number (%).
*The denominator for the AE proportions is 121 total AEs.
†The denominator for the SAE proportions is 13 total SAEs.
AE, adverse event; GCA, giant cell arteritis; SAE, serious adverse event; TCZ, tocilizumab.
In the group of 29 patients that received a first TCZ course lasting 9 to 12 months, 8 (27.6%) patients developed 19 adverse events. The rate (95% CI) of adverse events and serious adverse events exclusively related to TCZ per 100 patient-years in this group of 29 patients was 21 (9.1 to 41.4) and 5.2 (0.6 to 19.0), respectively. In the group of 36 patients that received a first TCZ course lasting over 12 months, 13 (36.1%) patients developed 21 adverse events after the initial 12 months of treatment. The rate (95% CI) of adverse events and serious adverse events exclusively related to TCZ per 100 patient-years in this group of 36 patients was 10 (4.8 to 18.3) and 1 (0.0 to 5.5), respectively.
Discussion
The optimal duration of TCZ treatment for patients with GCA has not been defined, and limited data are available regarding the efficacy and safety of long-term TCZ use in this patient population. Our results suggest that TCZ treatment beyond 12 months is efficacious in maintaining disease remission and sparing glucocorticoid use in most patients, with a long-term safety profile consistent with that observed with TCZ for other indications.18 19 In addition, more than 40% of patients who stopped TCZ due to long-term remission or adverse events after a mean time of 1.6 years of treatment subsequently relapsed, and longer TCZ courses were associated with decreased risk of relapse compared with shorter courses. Only one case of GCA-related permanent vision loss occurred during the follow-up time in a patient receiving TCZ.
The landmark clinical trials of TCZ in GCA used TCZ for a treatment duration of 12 months.12 13 Results from these trials and observational studies have shown that with the use of high doses of TCZ (ie, 162 mg weekly subcutaneously or 8 mg/kg monthly intravenously), 15%–30% of patients relapsed during treatment within 1 year.12–15 Furthermore, approximately 60% of patients in remission who discontinued TCZ by the end of the first year relapsed within the following 2 years.16 In the current study, most patients maintained their first TCZ treatment for more than 12 months, and 20% of them received more than one TCZ treatment course. In agreement with prior reports, relapse occurred in approximately 15% of patients while on their first TCZ course and in approximately 22% of patients when all TCZ courses were considered for each individual patient. In addition, our data showed that 40.8% of patients relapsed after stopping TCZ due to long-term remission or adverse events, with a Kaplan-Meier-estimated relapse rate of 47.3% at 18 months after drug discontinuation. Further research is needed to identify those individuals at greater risk of relapse after early TCZ discontinuation to maintain therapy beyond 12 months and to investigate whether a step-down strategy reducing the dose of TCZ prior to treatment withdrawal could improve the outcomes after therapy cessation.17 20 Until those data are generated, the duration of treatment for GCA patients should be individualised as recommended by major rheumatological societies based on several factors including but not limited to the patient’s clinical course (eg, relapsing vs non-relapsing), the occurrence of treatment-related adverse events, the clinical judgement of the treating physician and the patient’s preferences.21 22
Most patients were able to wean off glucocorticoids completely after the introduction of TCZ. However, unlike in clinical trials that employed 6 months of glucocorticoids in combination with TCZ,12 13 in this real-world cohort of patients, an average of 10 months of prednisone therapy was needed from the moment of TCZ initiation to prednisone discontinuation. Of note, most patients deemed to be in long-term remission (and therefore able to stop TCZ) by the treating rheumatologist had been off prednisone for a mean period of approximately 7 months before ceasing TCZ therapy.
As expected, most relapses occurring on TCZ demonstrated normal levels of the inflammatory markers CRP and ESR. However, a sizeable number of relapses developing after TCZ discontinuation exhibited normal inflammatory markers as well despite occurring in the absence of prednisone therapy in more than 90% of cases. This phenomenon was observed in particular with serum CRP, which was below 10 mg/L in approximately 70% of relapses off TCZ. These data are compatible with those of the GiACTA trial, in which approximately 60% of patients who stopped TCZ after achieving the primary endpoint of sustained glucocorticoid-free remission at week 52 exhibited normal CRP levels at the time of subsequent relapse.23 In our study, ESR also correlated poorly with disease activity after TCZ discontinuation, with approximately 60% of relapses after TCZ withdrawal presenting with an ESR below 30 mm/hour. The reason why a sizeable number of relapses after TCZ discontinuation occurred with normal inflammatory markers is unclear, but might be related with residual effects that IL-6 blockade could have in the liver synthesis of acute phase reactants. Nevertheless, further research is needed to draw conclusions. Altogether, these data indicate that until better biomarkers of disease activity are discovered, the diagnosis of GCA relapse during and after TCZ treatment should mostly rely on clinical grounds.
The adverse events related to glucocorticoids and TCZ in this cohort were consistent with the safety profile previously observed with these medications.3 24 25 Over an average of 3 years of follow-up, serious treatment-related complications occurred in approximately 14% of patients, with infection as the most frequent serious adverse event associated with TCZ and/or glucocorticoids. The initial TCZ course had to be discontinued due to treatment-related adverse events in nearly 10% of patients. Reassuringly, the rate of adverse events and serious adverse events exclusively related to TCZ declined over time.
This study has several strengths. To our knowledge, this is the first study to report detailed real-world data on the outcomes in patients treated with long-term TCZ and specifically address the effects of TCZ treatment duration on the risk of relapse and the incidence of relapse after discontinuation of TCZ courses longer than 12 months. An additional design-related strength is that each patient served as their own control because all patients were longitudinally evaluated during TCZ treatment and after TCZ discontinuation.
This study also had certain limitations. Because this was a retrospective assessment of real-world data, missing information and individual differences in the use of medications administered in routine clinical practice could have introduced bias. Incomplete documentation of data related to individual prednisone tapering regimens made the calculation of cumulative prednisone dose, a key outcome measure in GCA, inaccurate and therefore not analysable. This constraint was not surprising, given the extended length of prednisone tapering used in the treatment of GCA and the frequent dose modification that may not only occur during clinical visits but also in between visits. Yet, we were able to estimate important trends of glucocorticoid use during long-term TCZ treatment and observed, as expected, that most patients were able to discontinue prednisone by the end of the follow-up period. In addition, formal recommendations for vascular imaging in GCA were published in Europe in 201826 and in USA in 202122; therefore, vascular imaging was not obtained in the majority of this real-world cohort that was initiated in 2010. Unfortunately, the absence of systematic imaging acquisition precluded the assessment of long-term arterial damage and did not allow a meaningful analysis of the effects of large vessel involvement on other patient outcomes. Despite these limitations, our present findings expand the results from prior controlled clinical trials and observational studies and provide information that could be valuable for clinical decision making when considering the risks and benefits of TCZ continuation vs discontinuation beyond the arbitrary time point of 1 year.
In conclusion, long-term TCZ treatment was efficacious in maintaining disease remission and sparing the use of prednisone in most patients with GCA. After TCZ discontinuation due to long-term remission or adverse events, nearly 40% of patients relapsed. Patients discontinuing TCZ after 9–12 months demonstrated significantly greater risk of relapse compared with those continuing TCZ treatment beyond 12 months. A substantial number of relapses after TCZ cessation exhibited normal CRP and ESR levels. In this cohort of patients, the rate of adverse events and serious adverse events exclusively related to TCZ numerically declined over time.
Acknowledgments
The authors would like to thank Bongin Yoo for the careful review and feedback on the manuscript. Support for third-party editorial assistance, furnished by Nicola Gillespie, DVM, of Health Interactions, was provided by Genentech.
Footnotes
Contributors: MAM, JHS and SHU collected the data. All authors analysed and interpreted the patient data. All authors reviewed and approved the final manuscript. SHU is responsible for the overall content as the guarantor.
Funding: This study was funded by Genentech, Inc.
Competing interests: SHU has received research funding from Genentech. SVM and JHS are employees and shareholders of Genentech. ND and AP are consultants for Genentech. JHS is a consultant for and has received research funding from Roche. MAM has no disclosures.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Qualified researchers may request access to individual patient-level data through the clinical study data request platform (http://www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Partners Human Research Committee institutional review board (IRB) (Protocol #: 2017P001636) and was conducted in accordance with the Declaration of Helsinki. All data extracted from the EMRs were stored deidentified prior to the analysis. As per our institutional IRB guidelines, this retrospective research did not require informed consent.
References
- 1.Alba MA, García-Martínez A, Prieto-González S, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore) 2014;93:194-201. 10.1097/MD.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernández-Rodríguez J, García-Martínez A, Casademont J, et al. A strong initial systemic inflammatory response is associated with higher corticosteroid requirements and longer duration of therapy in patients with giant-cell arteritis. Arthritis Rheum 2002;47:29–35. 10.1002/art1.10161 [DOI] [PubMed] [Google Scholar]
- 3.Proven A, Gabriel SE, Orces C, et al. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003;49:703–8. 10.1002/art.11388 [DOI] [PubMed] [Google Scholar]
- 4.Jover JA, Hernández-García C, Morado IC, et al. Combined treatment of giant-cell arteritis with methotrexate and prednisone. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001;134:106–14. 10.7326/0003-4819-134-2-200101160-00010 [DOI] [PubMed] [Google Scholar]
- 5.Hoffman GS, Cid MC, Hellmann DB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002;46:1309–18. 10.1002/art.10262 [DOI] [PubMed] [Google Scholar]
- 6.Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001;19:495–501. [PubMed] [Google Scholar]
- 7.Leon L, Rodriguez-Rodriguez L, Morado I, et al. Treatment with methotrexate and risk of relapses in patients with giant cell arteritis in clinical practice. Clin Exp Rheumatol 2018;36:121–8. [PubMed] [Google Scholar]
- 8.Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum 2007;56:2789–97. 10.1002/art.22754 [DOI] [PubMed] [Google Scholar]
- 9.Langford CA, Cuthbertson D, Ytterberg SR, et al. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017;69:837–45. 10.1002/art.40044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venhoff N, Schmidt W, Bergner R, et al. Secukinumab in giant cell arteritis: a randomized, parallel-group, double-blind, placebo-controlled, multicenter phase 2 trial (abstract). Arthritis Rheumatol 2021;73:L19. [Google Scholar]
- 11.Cid MC, Unizony SH, Blockmans D, et al. Efficacy and safety of mavrilimumab in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2022;81:653–61. 10.1136/annrheumdis-2021-221865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28. 10.1056/NEJMoa1613849 [DOI] [PubMed] [Google Scholar]
- 13.Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016;387:1921–7. 10.1016/S0140-6736(16)00560-2 [DOI] [PubMed] [Google Scholar]
- 14.Unizony SH, Bao M, Han J, et al. Treatment failure in giant cell arteritis. Ann Rheum Dis 2021;80:1467–74. 10.1136/annrheumdis-2021-220347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unizony S, McCulley TJ, Spiera R, et al. Clinical outcomes of patients with giant cell arteritis treated with tocilizumab in real-world clinical practice: decreased incidence of new visual manifestations. Arthritis Res Ther 2021;23:8. 10.1186/s13075-020-02377-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone JH, Han J, Aringer M, et al. Long-term effect of tocilizumab in patients with giant cell arteritis: open-label extension phase of the Giant Cell Arteritis Actemra (GiACTA) trial. Lancet Rheumatol 2021;3:e328–36. 10.1016/S2665-9913(21)00038-2 [DOI] [PubMed] [Google Scholar]
- 17.Tomelleri A, Campochiaro C, Sartorelli S, et al. POS0266 Effectiveness of a spacing-up strategy after one-year course of weekly tocilizumab in patients with giant cell arteritis: a single-centre prospective study. Ann Rheum Dis 2022;81:375–6. 10.1136/annrheumdis-2022-eular.784 [DOI] [Google Scholar]
- 18.Sepriano A, Kerschbaumer A, Smolen JS, et al. Safety of synthetic and biological DMARDs: a systematic literature review Informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2020;79:760–70. 10.1136/annrheumdis-2019-216653 [DOI] [PubMed] [Google Scholar]
- 19.Nishimoto N, Miyasaka N, Yamamoto K, et al. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis 2009;68:1580–4. 10.1136/ard.2008.092866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderón-Goercke M, Loricera J, Moriano C, et al. Optimisation of tocilizumab therapy in giant cell arteritis. A multicentre real-life study of 471 patients. Clin Exp Rheumatol Published online November 2, 2022. 10.55563/clinexprheumatol/oqs8u9 [DOI] [PubMed] [Google Scholar]
- 21.Scolnik M, Brance ML, Fernández-Ávila DG, et al. Pan American League of Associations for Rheumatology guidelines for the treatment of giant cell arteritis. Lancet Rheumatol 2022;4:e864–72. 10.1016/S2665-9913(22)00260-0 [DOI] [PubMed] [Google Scholar]
- 22.Maz M, Chung SA, Abril A, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of giant cell arteritis and takayasu arteritis. Arthritis Rheumatol 2021;73:1349–65. 10.1002/art.41774 [DOI] [PubMed] [Google Scholar]
- 23.Unizony S, Mohan SV, Han J, et al. POS0808 Characteristics of giant cell arteritis flares after successful treatment with tocilizumab: results from the long-term extension of a randomized controlled phase 3 trial. Ann Rheum Dis 2021;80:E0516. 10.1136/annrheumdis-2021-eular.2602 [DOI] [Google Scholar]
- 24.Genentech, Inc . ACTEMRA prescribing information. South San Francisco, CA, 2022. [Google Scholar]
- 25.Wilson JC, Sarsour K, Collinson N, et al. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): A nested case-control analysis. Semin Arthritis Rheum 2017;46:819–27. 10.1016/j.semarthrit.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 26.Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43. 10.1136/annrheumdis-2017-212649 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. Qualified researchers may request access to individual patient-level data through the clinical study data request platform (http://www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available here: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.