Abstract
Introduction
Empirical studies on effective communication for amyloid disclosure in mild cognitive impairment (MCI) are lacking. We aimed to study the impact of six communication strategies.
Method
We performed a randomized controlled trial with seven randomly assigned, video‐vignette conditions: six emphasizing a communication strategy and one basic condition. All showed a scripted consultation of a neurologist disclosing positive amyloid positron emission tomography (PET) scan results to an MCI patient. Healthy individuals (N = 1017; mean age ± SD 64 ± 8, 808 (79%) female) were instructed to imagine themselves in the video, answered questionnaires assessing information recall, emotional state, and behavioral intentions, and evaluate the physician/information.
Results
“Risk best practice” resulted in highest free recall compared to other strategies (P < .05), except “emotional support”. Recall in “emotional support” was better compared to “basic‐‘ and elaborate information”(P < .05). “Risk best practice” resulted in the highest uncertainty (P < .001). “Teach‐back” and “emotional support” contributed to the highest evaluations (P ‐values < .01).
Conclusion
Risk communication best practices, attending to emotions, and teach‐back techniques enhance information recall of amyloid‐PET results, and could contribute to positive care evaluations.
Keywords: amyloid status, communication, disclosure, MCI, mild cognitive impairment
1. INTRODUCTION
Timely detection of Alzheimer's disease (AD) is important, for example, for disease (self) management and prevention efforts. 1 New diagnostic tests, such as amyloid positron emission tomography (PET), can contribute to early and accurate diagnosis of AD. This is especially relevant in individuals with mild cognitive impairment (MCI), who show cognitive decline without meeting criteria for dementia. Roughly 50% of MCI patients develop dementia in 3 years. 2 Amyloid‐positive MCI patients are more likely to progress to dementia. 3
Communicating amyloid‐PET results, including the risk of dementia, to MCI patients can be challenging. The predictive value of a positive amyloid status is not perfect and the time to dementia varies. Because of these uncertainties, physicians experience difficulty in providing MCI patients with results of an amyloid‐PET scan and report concerns about patients’ recall of this complex information and the emotional impact thereof. 4
Available protocols for disclosure of amyloid‐PET results have been developed in the context of research and are based on expert opinion rather than on empirical data. 2 , 5 , 6 Disclosure protocols propose that a disclosure consultation should be done in‐person, include the dementia risk, and make use of visual aids (eg, by using the patient's PET scan). 2 , 5 , 6 However, studies on the effectiveness of such strategies are lacking. Communication strategies for uncertain or complex messages have been studied empirically in other fields, such as oncology, and include, for example, providing emotional support and specific and defined risk information as best‐practice recommendation. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 If such strategies improve information recall, without adversely impacting emotions, they may lead to additional benefits such as a positive evaluation of the physician. 15 , 16
RESEARCH IN CONTEXT
Systematic Review: The authors searched traditional sources for literature (eg, PubMed). Previous literature has established concerns about understanding and emotional impact of an amyloid‐positive imaging result in individuals with mild cognitive impairment (MCI), and has developed expert‐based frameworks for disclosure. However, empirical studies on communicating a positive amyloid‐imaging result are lacking. These relevant publications are appropriately cited.
Interpretation: We evaluated the effect of seven different communication strategies in a randomized controlled trial with an online video‐vignette design. Video vignettes showed a scripted consultation in which a neurologist discloses a positive amyloid positron emission tomography (PET) scan result to an MCI patient. We included 1017 healthy individuals who were instructed to imagine themselves in the situation of the video‐vignette patient. Participants answered questionnaires before and after viewing one of the seven randomly assigned video vignettes. Completed questionnaires measured information recall, emotional state, behavioral intentions, trust in and satisfaction with the physician, and information satisfaction.
Future Directions: The results reported in this article provide the first steps toward evidence‐based recommendations for communication strategies that are best suited to disclose a positive amyloid status to MCI patients. These results generate new hypotheses, examples of which include: (1) examining moderation effects on information recall in MCI patients of individual characteristics such as age, health literacy, or coping style; or (2) examining possible cumulative benefits of communication strategies.
The aim of the present study was to investigate how seven commonly recommended communication strategies used when disclosing positive amyloid‐PET results impact outcomes in terms of (1) information recall, (2) emotional state, (3) behavioral intentions, and (4) experiences/evaluations.
2. METHODS
2.1. Study design and experimental conditions
We performed a randomized controlled trial (RCT) using an experimental video‐vignette design. 17 , 18 , 19 Elements of physician communication were varied systematically across seven, otherwise standardized, scripted videotaped consultations (video vignettes) that were developed following published recommendations. 17 , 18 These video vignettes, made with professional actors, depict a consultation in which a neurologist communicates the results of a positive amyloid‐PET scan and the subsequent increased risk for developing dementia to a patient with MCI and his daughter. Average video‐vignette time was 8 minutes and 27 seconds. Explanation of all seven conditions and a detailed description of the vignettes is provided in Table 1. The first row comprises the “Basic information” condition and describes the generic content of the consultation. The other rows describe the communication elements that were added or omitted in the other six conditions in comparison to the “Basic information” condition. Communication of amyloid status occurred face‐to‐face in all communication strategies. In addition, two of the seven communication strategies included a visual aid to support communication.
TABLE 1.
Video‐vignette communication elements included per condition
| Condition | Elements in condition |
|---|---|
|
C1: Basic information |
|
|
C2: Elaborate information |
|
|
C3: Non‐specified risk |
|
|
C4: Risk best practice |
|
|
C5: Visual PET‐scan |
|
|
C6: Teach‐back |
|
|
C7: Emotional support
Based on:
|
|
The base script was developed based on current practice, by listening to audio recordings of real consultations, recorded in eight different memory clinics across The Netherlands. 65
Through the predictive models made by van Maurik et al., in combination with the ADappt tool, a specific risk estimate was made possible. 3 , 66 Currently in clinical practice, a specific risk estimate is not used; nor is a specific time‐frame. However, for the present study, it was necessary to include both a specific risk estimate, and a specific time‐frame, since: (1) differences in the information provided would be too great between conditions otherwise, and (2) health‐risk information and best practices on how to explain health risks require a frame of reference to interpret the health risk, that is, a specific risk estimate as well as a time‐frame (3 years).
Experts through experience (ie, MCI patients and care partners of patients with MCI and dementia); experts in the field of video‐vignette design or communication research; experts through knowledge (ie, neurologists, colleagues in the field of dementia, and [medical‐] communication experts).
The study was reviewed by the board of the medical ethics committee of Amsterdam University Medical Centers (Amsterdam UMC), location VU Medical Center (VUmc). Prior to the start of the study, the RCT was registered at trialregister.nl, the Dutch Trial Registry (Trial NL7222). All participants gave informed consent.
2.2. Participants and procedures
In this study, participants in the same age group as real patients acted as analogue patients, 17 , 18 , 19 , 20 that is, as proxies for real patients, and were instructed to imagine themselves in the position of the patient in the video while viewing one of the seven video vignettes to which they were assigned through simple randomization. Individuals were considered eligible if they were 50 years or older, had no self‐reported diagnosis of a neurodegenerative disease, and had no memory complaints for which they would want to seek, or had sought, professional medical help. Participants were excluded if they had audiovisual impairments, did not master the Dutch language, or did not have access to Internet. Eligible individuals (N = 5740) were invited to participate between January and December 2019, through the Dutch Brain Research Registry (www.hersenonderzoek.nl). 21 Individuals who demonstrated interest (N = 2514) received a personal link to the online questionnaire, which also comprised the assigned video vignette. Of those interested individuals, 1328 started the online questionnaire and complete data of 1017 participants were available for analysis.
2.3. Outcome measures
Participants completed questionnaires immediately before (T0) and after (T1) viewing the video vignette (Figure 1). Primary outcome was information recall. Secondary outcomes included emotional response (the change in state emotions from T0 to T1), behavioral intentions, and evaluation of the physician and the provided information.
FIGURE 1.
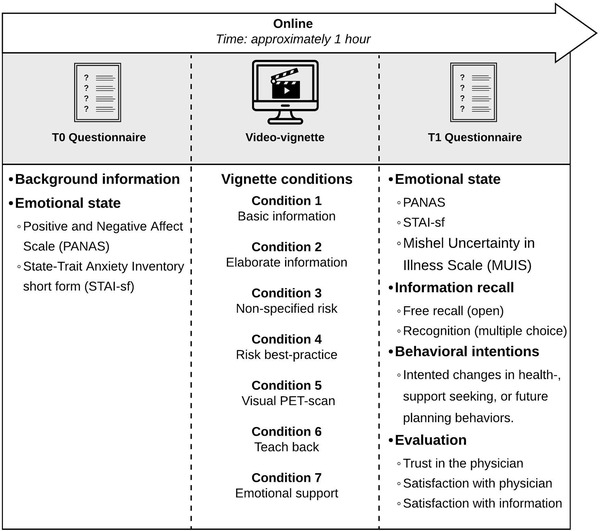
Schematic overview of study measures
FIGURE 2.
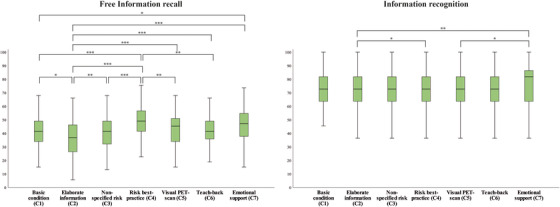
Boxplots present significant differences between communication strategies on free information recall, and information recognition. Analyses of variance (ANOVAs) showed an effect of condition on both free information recall and information recognition. Post hoc Bonferroni adjusted comparisons between conditions showed highest free information recall in the risk‐best practice condition, and highest information recognition in the emotional support condition. * P < .05, ** P < .01, P < .001
2.3.1. Primary outcome measures
Information recall was operationalized as free information recall and recognition of information provided by the physician in the video consultation and assessed with a study‐specific questionnaire. The questionnaire was developed based on the video‐ vignette content and piloted among 10 individuals from the target population. Participants answered 11 open‐ended questions (free recall), and consecutively the same 11 questions in multiple‐choice format (recognition). A coding scheme was developed to score the free‐recall answers, using directed content‐coding analysis (Table S1). 22 , 23 , 24 Free‐recall sum scores were calculated based on points assigned for correct elements per answer (range 0‐26.5), after which this sum score was transformed to range from 0 to 100 (percentage correct). For multiple‐choice questions, participants were instructed to select the correct answer from four possible answers. Recognition sum scores were calculated by summing correct answers (range 0‐11), after which they were transformed to a 0 to 100 scale (percentage correct).
2.3.2. Secondary outcome measures
Emotional state was assessed at T0 and T1 using the Dutch version of the Positive and Negative Affect Scale (PANAS), 25 and the Dutch version of the short‐form State‐Trait Anxiety Inventory (S‐STAI‐S). 26 , 27 Emotional response to the vignette was then calculated as a change in emotions over time (∆), that is, T1 minus T0, such that a positive delta indicates an increase in emotions. Self‐reported uncertainty with regard to the video‐patients’ health and disease status was assessed at T1 using a selection of 10 items from the Mishel Uncertainty in Illness Scale (MUIS; Cronbach α = .61). 28
Behavioral intentions, that is, how likely a participant would be to change certain health, support‐seeking, or future‐planning behaviors were assessed with a study‐specific questionnaire consisting of 11 items answered on a 7‐point Likert scale (1 = very unlikely to 7 = very likely; Cronbach α = .69). Evaluation of the video‐physician was measured by means of the Patient Satisfaction Questionnaire (PSQ‐5), on visual analog scales (ranging from 0 = not at all satisfied to 10 = very satisfied). 29 , 30 , 31 Trust in the video‐physician was assessed using an adapted version of the Trust in Oncologist Scale (TiOS‐sf), 32 and provided information was evaluated using a selection of nine items of the European Organization for Research and Treatment for Cancer information questionnaire (EORTC info25). 33 Higher scores indicated greater satisfaction, trust, and information satisfaction, respectively.
Background characteristics included age, sex, education level, and two questions on self‐reported medical or dementia knowledge using a 5‐point Likert scale (1 = no knowledge at all to 5 = a lot of knowledge).
2.3.3. Video‐vignette design validity check
We checked the degree to which participants could engage in the video vignette using the Video Engagement Scale (VES), which proved to be satisfactory in comparison to other video‐vignette studies (mean VES score ± SD 4.3 ± 1.3, on a scale from 1 to 7; see Table 2). 34 In addition, we checked the perceived realism of the video‐physician and depicted situation, and perceptions of communication strategies (15 items on a scale ranging from 0 = do not agree to 10 = agree completely). Perceived video‐physician (8.0 ± 1.7) and video‐situation realism (7.7 ± 2.0) was high, and communication strategies were all perceived as intended (Table 2).
TABLE 2.
Participant characteristics and mean scores (SD) of primary and secondary outcome measures per communication strategy condition
| Total Group | Basic information (C1) | Elaborate information (C2) | Non‐specified risk (C3) | Risk‐ Best practice (C4) | Visual PET scan (C5) | Teach‐ back (C6) | Emotional support (C7) | |
|---|---|---|---|---|---|---|---|---|
| N = 1017 | N = 134 | N = 144 | N = 134 | N = 160 | N = 141 | N = 149 | N = 155 | |
| Sex, F, n (%) | 808 (79%) | 108 (81%) | 110 (76%) | 115 (86%) | 121 (76%) | 109 (77%) | 121 (81%) | 124 (80%) |
| Age (years) | 63.7 (8.0) | 64.1 (7.2) | 64.0 (8.4) | 63.9 (8.3) | 63.1 (8.1) | 63.4 (8.7) | 63.4 (8.0) | 63.8 (7.6) |
| Years of education | 11.8 (3.1) | 11.6 (3.0) | 11.9 (3.0) | 12.2 (3.3) | 11.4 (3.1) | 11.6 (3.0) | 12.2 (3.2) | 11.9 (2.9) |
| Dementia patient caregiver b | 402 (40%) | 58 (43%) | 63 (44%) | 58 (43%) | 58 (36%) | 52 (37%) | 54 (36%) | 59 (38%) |
| Medical knowledge c , h (1‐5) | 3.0 (0.7) | 2.9 (0.7) | 3.0 (0.7) | 3.0 (0.7) | 3.0 (0.7) | 3.0 (0.7) | 3.0 (0.7) | 3.0 (0.7) |
| Dementia knowledge c , h (1‐5) | 2.9 (0.7) | 2.8 (0.7) | 2.8 (0.7) | 2.9 (0.7) | 2.9 (0.7) | 2.9 (0.8) | 2.9 (0.7) | 2.8 (0.8) |
| Perceived realism video‐physician d , h (0‐10) | 8.0 (1.7) | 8.2 (1.6) | 8.0 (1.5) | 8.0 (1.7) | 7.9 (1.6) | 7.9 (1.8) | 8.1 (2.0) | 8.0 (1.8) |
| Perceived realism video‐situation d , h (0‐10) | 7.7 (2.0) | 7.9 (1.7) | 7.5 (2.2) | 7.7 (2.0) | 7.6 (2.0) | 7.8 (1.9) | 8.0 (2.0) | 7.7 (2.0) |
| Video Engagement Scale e , h (VES; 1‐7) | 4.3 (1.3) | 4.3 (1.2) | 4.2 (1.3) | 4.4 (1.4) | 4.2 (1.2) | 4.4 (1.2) | 4.6 (1.3) | 4.2 (1.2) |
|
Free Recall i (0‐100) |
42.7 (12.1) a | 41.0 (10.7) | 36.4 (12.7) | 41.5 (11.2) | 48.1 (12.0) | 42.7 (11.5) | 42.5 (10.7) | 45.3 (12.6) |
| Recognition i (0‐100) | 73.9 (14.3) a | 72.9 (13.0) | 70.6 (14.7) | 74.8 (14.0) | 75.8 (13.6) | 71.1 (15.5) | 75.2 (14.2) | 76.2 (14.0) |
| PANAS (positive emotions ∆ j ) (−40 to 40) | −2.4 (5.4) | −2.1 (5.2) | −2.9 (6.0) | −2.6 (5.2) | −1.6 (5.1) | −2.8 (5.8) | −2.6 (5.4) | −2.5 (5.3) |
| PANAS (negative emotions ∆ j ) (−40 to 40) | 0.8 (4.7) | 0.6 (4.1) | 1.2 (4.6) | 1.4 (5.5) | 0.06 (4.5) | 1.2 (4.5) | 0.9 (5.1) | 0.6 (4.7) |
| STAI (anxiety ∆ j ) (−3 to 3) | 0.2 (0.5) | 0.2 (0.5) | 0.2 (0.5) | 0.3 (0.6) | 0.1 (0.5) | 0.2 (0.5) | 0.2 (0.6) | 0.1 (0.5) |
| MUIS f , h (uncertainty) (1‐5) | 2.1 (0.6) a | 2.0 (0.6) | 2.0 (0.6) | 2.1 (0.6) | 2.3 (0.6) | 2.1 (0.6) | 1.9 (0.6) | 2.0 (0.6) |
| Behavioral intentions g , h (1‐7) | 4.9 (0.8) | 5.1 (0.7) | 4.9 (0.8) | 5 (0.8) | 4.9 (0.8) | 4.9 (0.8) | 4.9 (0.8) | 4.9 (0.7) |
| Trust in physician h (1‐5) | 3.6 (0.8) a | 3.6 (0.8) | 3.5 (0.8) | 3.4 (0.9) | 3.5 (0.8) | 3.5 (0.8) | 3.7 (0.9) | 3.7 (0.8) |
| Satisfaction with physician h (0‐10) | 6.0 (2.0) a | 6.1 (1.8) | 5.8 (2.0) | 5.7 (2.1) | 5.7 (2.0) | 5.6 (1.9) | 6.4 (2.1) | 6.7 (2.0) |
| Satisfaction with information h (1‐4) | 2.1 (0.6) a | 2.2 (0.6) | 2.2 (0.6) | 2.0 (0.5) | 2.1 (0.5) | 2.1 (0.5) | 2.3 (0.5) | 2.1 (0.6) |
Note: Table shows mean (SD), unless otherwise specified. Ranges indicate minimum score to maximum score for all reported variables.
ANOVA showed significant group differences (P < .05); post hoc comparisons between groups are shown in Figures 2 and 3.
Dementia patient caregiver indicates number of participants who were actively a caregiver, or have been in the past, for an individual with dementia.
Medical knowledge and dementia knowledge are self‐reported levels of knowledge.
Perceived physician realism and situation realism are self‐reported levels of the realism of the video‐vignette physician and the situation/scenario displayed.
Scores on the VES proved to be satisfactory in comparison to other video‐vignette studies. 34
Higher scores indicate more uncertainty on the MUIS (Mishel Uncertainty in Illness Scale).
Higher scores indicate more reported intended behavior changes if the participant would find himself/herself in the video‐patient's situation; lower scores indicate less or no intended changes to behaviors.
Presented scores are average scores.
Presented scores are sum totals and indicate percentage correct.
Presented scores are delta scores (∆), indicating a change in emotions over time. Here, a positive ∆ indicates an increase in experienced emotions over time, and a negative ∆ indicates a decrease in experienced emotions (∆ = T1‐T0).
2.4. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 25 for Windows (IBM Corp., Armonk, NY). Demographic characteristics were compared between conditions using analysis of variance (ANOVA), or Kruskal‐Wallis tests, where appropriate. Information free recall and recognition scores, and participants’ information‐ and physician‐evaluation scores were compared between conditions using univariate ANOVAs with Bonferroni post hoc test. Changes in emotional state over time between conditions were analyzed using ANOVAs for repeated measures with Bonferroni post hoc tests. P‐values < 0.05 were considered statistically significant.
3. RESULTS
3.1. Demographics
Our sample of 1017 participants (808 [79%] female) was on average (mean age ± SD) 63.7 ± 8.0 years old, had 11.8 ± 3.1 years of education, and comprised 402 (40%) (former) care partners for an individual with dementia. Self‐reported medical (3.0 ± 0.7), and dementia knowledge (2.9 ± 0.7) was average. Participant characteristics are listed in Table 2. We found no differences across conditions in sex, age, education, prior medical or dementia knowledge, former care‐partner status, video engagement, or perceived video‐vignette realism. Therefore, randomization was considered successful, and no covariates were included in further analysis.
3.2. Information recall and recognition
Means and SDs are provided in Table 2. ANOVAs showed differences between communication strategies on free information recall (F(6,1010) = 14.7, P < .001; Figure 3). Post hoc Bonferroni‐adjusted tests showed that the “risk best practice” strategy (C4; mean % ± SD: 48.1 ± 12.0) resulted in the highest percentage free recall compared to all other communication strategies (P < .001‐P = .002), except “emotional support” (C7; P = .783). Recall in “emotional support” (C7; 45.3 ± 12.6) was better compared to the “basic information provision” (C1, 41.0 ± 10.7; P = .040), and “elaborate information” communication strategies (C2, 36.4 ± 12.7; P < .001). Free recall was lowest in the “elaborate information” condition (P‐values ranging from < 0.05 for all other strategies).
FIGURE 3.
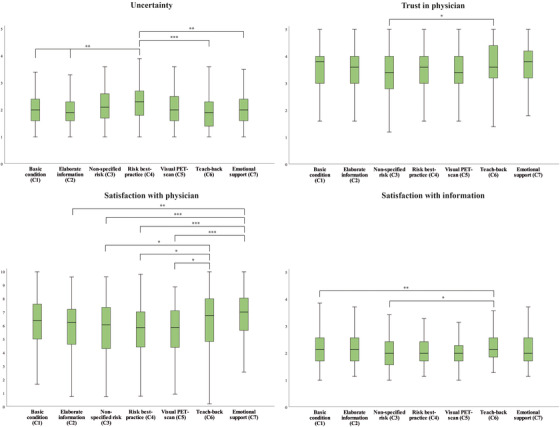
Boxplots present significant differences between communication strategies on outcome measures uncertainty, trust, satisfaction with physician, and information satisfaction. Analysis of variance (ANOVA) showed an effect of conditions for uncertainty, trust in the physician, satisfaction with the physician, and satisfaction with the information. Post hoc Bonferroni adjusted comparisons between conditions showed highest uncertainty in the risk‐best practice condition, highest trust in‐ and satisfaction with the physician, and the information in the teach‐back condition. * P < .05, ** P < .01, P < .001
In addition, we found a main effect of communication strategy on information recognition (F(6, 1010) = 3.8, P = .001). Inspection of post hoc results highlights the same conditions as reported above (Figure 3). Participants in the “risk best practice” condition (C4; 75.8 ± 13.6) recognized more information than participants in the “elaborate information” condition (C2, 70.6 ± 14.7; P = .033). In addition, “emotional support” (C7; 76.2 ± 14.0) resulted in higher information recognition scores compared to “elaborate information provision”(C2, 70.6 ± 14.7; P = .015), and visual support by showing the PET scan (C5, 71.1 ± 15.5; P = .039).
3.3. Secondary outcome measures
Using ANOVA for repeated measures, we observed an overall decrease in positive affect over time (PANAS positive scale main effect time ∆ −2.4 ± 5.4; P < .001), but no main effect of condition or interaction between time and condition (F(6, 1010) = 1.1, Pfor interaction = .358). For negative affect, we observed an increase over time (PANAS negative scale ∆ 0.8 ± 4.7; P < .001), but also no main effect of condition or interaction between time and condition (F(6, 1010) = 1.4, Pfor interaction = .195). Likewise, anxiety increased over time (STAI ∆ 0.2 ± 0.5; P < .001), but no main effect of condition or interaction between time and condition was found (F(6, 1010) = 1.8, Pfor interaction = .092).
ANOVA showed a main effect of communication strategy on uncertainty (F(6,1008) = 5.7, P < .001). Participants who viewed the “risk best practice” communication strategy (C4; 2.3 ± 0.6) experienced more uncertainty concerning the video‐patients’ health and disease status compared to all other communication strategies (P < .001 to P = .015), except for the “non‐specified risk” communication strategy (C3; 2.1 ± 0.6), and the “visual PET scan” strategy (C5; 2.1 ± 0.6), which had intermediate levels of uncertainty.
Communication strategy also had an effect on trust in the physician (F(6,1010) = 2.9, P = .008), as participants in the “teach‐back” communication strategy (C6, 3.7 ± 0.9; P = .05) reported more trust in the physician compared to the “non‐specified risk” strategy (C3; 3.4 ± 0.9). In addition, self‐reported satisfaction with the video‐physician was affected by communication strategy (F(6,1010) = 6.7, P = .004). Participants in the “emotional support” condition (C7; 6.7 ± 2.0), and “teach‐back” condition were most satisfied with the video‐physician compared to the other strategies (C3, 5.7 ± 2.1; Figure 3). Satisfaction with the provided information also differed between communication strategies (F(6, 1010) = 3.2, P = .004). Participants in the “teach‐back” condition (C6; 2.3 ± 0.5) were more satisfied with the information compared to participants in the “non‐specified risk” condition (C3, 2 ± 0.5; P = .004), and the “visual PET scan” condition (C5, 2.1 ± 0.5; P = .030). Communication strategy had no effect on behavioral intentions (F(6, 1010) = 1.6, P = .137).
4. DISCUSSION
This randomized, experimental video‐vignette study provides initial steps toward evidence‐based recommendation for communication strategies for disclosure of a positive amyloid‐PET result to MCI patients. Explicitly mentioning the risk, with both positive and negative framing (ie, risk best practice), supplemented with actively attending to emotions, and use of the teach‐back method to check understanding, maximized information uptake without adversely impacting emotions. Moreover, this resulted in higher trust and satisfaction.
An increasing number of patients with MCI want to know their amyloid status. 4 Moreover, communication of amyloid status will become even more relevant when disease‐modifying treatments become available. Thus, evidence‐based communication strategies are necessary to ensure that patients understand what a positive amyloid status entails, and what it means with regard to their risk for developing dementia. One of physicians’ concerns is whether MCI patients correctly understand the complicated message of a positive amyloid scan. 35 We show that the use of risk best‐practice recommendations maximizes information uptake in older adults. Risk best practice, which has been developed in the context of for example cancer, 7 , 8 includes visualization of risk (eg, by bar graph or icon array); neutral framing (equal focus on both potential outcomes, ie, 70% will develop dementia and 30% will not develop dementia); and use of natural frequencies (eg, 70 of 100 people, rather than “high risk”). We observed more uncertainty immediately after viewing the video vignette for the risk best practice communication strategy. Apparently, better understanding comes with more experienced uncertainty about the disease and future development. In fact, this is in line with the nature of the conveyed message (increased risk of dementia, not a certainty). However, uncertainty might benefit patients too. For example, uncertainty may leave room for hope, providing a way of coping with a potentially negative outcome. 36 , 37
Dementia is one of the more feared diagnoses in older age. 38 Therefore, it is of great importance, but challenging, to ensure a correct understanding of what a positive amyloid status means in MCI. According to the theory of attentional narrowing, experiencing emotional distress requires attention, thus restricting available attention for additional informational sources. In turn, this may affect information processing, resulting in poorer retention of information. 39 , 40 , 41 The current study shows how empathic physician behavior, that is, attending to emotions, may mitigate the negative effect of attentional narrowing on information recall. Similar effects of empathic physician behavior on information recall have been shown previously, although results have been mixed. 42 , 43 Possibly, empathic physician behavior works as a buffer against adverse emotional effects when receiving an emotionally charged message, thus mitigating the attentional narrowing effect. However, a potential buffer effect of empathic behavior on information recall may be limited to actual, real medical consultations in which experienced emotions are more severe.
Perhaps counterintuitively, providing more elaborate information to fully explain amyloid and its role in AD does not equate to higher information recall about these mechanisms, or to trustworthiness or satisfaction. On the contrary, more elaborate information resulted in less information recalled compared to all other communication strategies. This suggests that providing more elaborate information without taking specific risk‐communication strategies into account is not the best method. Providing more elaborate information could even result in information overload. 44 , 45 , 46 Recommendations in expert opinion–based protocols advise providing elaborate information about amyloid to patients undergoing amyloid testing, preferably pre‐disclosure. 5 , 6 In addition, the modality of delivering relevant information may be relevant (eg, verbal, written, or audio‐visual). For example, there is a growing body of evidence supporting the benefits of providing health information in an audio‐visual format. 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 Thus, timing of providing the necessary information warrants further investigation, in addition to modality of the information.
Our results show that attending to emotions as well as using the teach‐back strategy result in a more positive evaluation of the physician, which may contribute to a positive physician‐patient relationship. Here, the teach‐back strategy consisted of the physician asking the patient to repeat the provided information in their own words, to ensure that the patient recalled the information correctly. Perhaps the teach‐back interaction is viewed as another form of empathic behavior, thus resulting in a positive evaluation. Current literature suggests that a good physician‐patient relationship benefits patients in several ways, such as desirable behavioral changes, or improved patient‐participation during consultations. 58 , 59 , 60 Furthermore, establishing a good physician‐patient relationship is considered essential to high‐quality health care. 61
A strength of this study is the use of a randomized controlled experimental video‐vignette design, which enabled a large study sample size and allows for drawing conclusions about the causality (ie, direction) of effects. This enabled us to empirically study communication strategies, resulting in specific recommendations. This study also has some limitations. Although previous research indicates that analogue patients can be used validly as a proxy to real patients to evaluate physicians’ communication behavior in a video‐vignettes design, 19 , 20 this approach may limit the ecological validity of the results, particularly given that we included cognitively healthy individuals, whereas MCI patients are cognitively impaired. For example, one might wonder if recall levels (36%‐48% in our sample) are comparable to clinical reality, although these recall percentages are comparable to findings in observational research. 62 , 63 , 64 Similarly, it is difficult to say whether the lack of effect on behavioral intentions is due to the use of analogue patients in this video‐vignette design or to alternative explanations. For example, there might have been a lack of variation in behavioral intentions, thereby diminishing our chances to find an effect of communication on behavioral intentions, because many individuals may feel no need to make changes to their behavior because they might feel they already live healthy and/or are well prepared for the future; or because they feel changing their behavior may serve no real purpose. Moreover, experimental video‐vignette studies are designed to find proof of principles. Our study provides merely the first insights required toward developing specific recommendations regarding use of communication strategies in amyloid‐imaging results disclosure. The next step is validation of these results in a prospective research design among MCI patients. In addition, the current study prioritized information recall/recognition as primary outcome measure, although a different primary focus may be equally valuable to different stakeholders. Therefore, it might be worth considering including the views of clinicians, patients, or care partners in the design of future studies to determine primary outcome measures. In addition, more than three‐fourths of our participants were women. However, there were no gender differences between conditions in our study, and we therefore believe it is unlikely that this influenced our results. Finally, we limited our investigation to the direct effects of communication strategies on all outcome measures individually, not incorporating potential moderating or mediating effects, for example, of participant characteristics like age, health literacy, or preferred coping style. However, individual differences may play a role in information processing.
4.1. Conclusion
This study provides the first insights required toward development of evidence‐based recommendations for communication strategies best suited to disclose a positive amyloid status to persons with MCI. Use of risk communication best practices and actively attending to emotions enhances information recall, without adversely impacting emotions. In addition, actively attending to emotions and incorporating teach‐back techniques contribute to a positive evaluation of the disclosure consultation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGEMENTS
The authors would like to thank Annelies van der Vlies, Hanneke Rhodius, and all participants and physicians for their contribution to this study. Participant recruitment was accomplished through Hersenonderzoek.nl, a Dutch online registry that facilitates participant recruitment for neuroscience studies (www.hersenonderzoek.nl). Hersenonderzoek.nl is funded by ZonMw‐Memorabel (project no. 73305095003), a project in the context of the Dutch Deltaplan Dementie, Gieskes‐Strijbis Foundation, the Alzheimer's Society in The Netherlands, and Brain Foundation Netherlands. The Alzheimer center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. Wiesje M. van der Flier, Ellen M.A. Smets, NV, and Philip Scheltens are recipients of ABOARD, which is a public‐private partnership receiving funding from ZonMW (#73305095007) and Health∼Holland, Topsector Life Sciences & Health (PPP‐allowance; #LSHM20106). PS, Wiesje M. van der Flier, and Leonie N.C. Visser are recipients of the EU Joint Programme ‐ Neurodegenerative Disease Research (JPND) project EURO‐FINGERS (ZonMW‐Memorabel #733051102). This project is supported through the following funding organizations under the aegis of JPND ‐ www.jpnd.eu: Finland, Academy of Finland; Germany, Federal Ministry of Education and Research; Spain, National Institute of Health Carlos III; Luxemburg, National Research Fund; Hungary, National Research, Development and Innovation Office; The Netherlands, Netherlands Organisation for Health Research and Development; Sweden, Swedish Research Council. WvdF is recipient of a grant by Stichting LSH‐TKI (ABIDE‐communication: LSHM16025), a collaboration project co‐financed by Alzheimer Nederland, Piramal Neuroimaging, VU Medical Center, and Amsterdam Medical Center, and financed by the Ministry of Economic Affairs and Climate Policy by means of the PPP Allowance made available by the Top Sector Life Sciences & health to stimulate public‐private partnerships. The chair of Wiesje van der Flier is supported by the Pasman stichting. PS is recipient of JPND‐EURO‐FINGERS (ZonMW #733051102). LNCV is supported by a fellowship grant received from Alzheimer Nederland (WE.15‐2019‐05). Hersenonderzoek.nl is supported by a grant from ZonMW Memorabel (#73305095003).
Fruijtier AD, van der Schaar J, van Maurik IS, et al. Identifying best practices for disclosure of amyloid imaging results: A randomized controlled trial. Alzheimer's Dement. 2023;19:285–295. 10.1002/alz.12630
REFERENCES
- 1. Dubois B, Padovani A, Scheltens P, Rossi A, Dell'Agnello G. Timely diagnosis for Alzheimer's disease: a literature review on benefits and challenges. J Alzheimers Dis. 2016;49:617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Maurik IS, Zwan MD, Tijms BMet al. Interpreting biomarker results in individual patients with mild cognitive impairment in the Alzheimer's Biomarkers in Daily Practice (ABIDE) project. JAMA Neurol. 2017;74:1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kunneman M, Pel‐Littel R, Bouwman FH, et al. Patients' and caregivers' views on conversations and shared decision making in diagnostic testing for Alzheimer's disease: the ABIDE project. Alzheimers Dement (N Y). 2017;3:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grill JD, Apostolova LG, Bullain S, et al. Communicating mild cognitive impairment diagnoses with and without amyloid imaging. Alzheimers Res Ther. 2017;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lingler JH, Butters MA, Gentry AL, et al. Development of a standardized approach to disclosing amyloid imaging research results in mild cognitive impairment. J Alzheimers Dis. 2016;52:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence‐based risk communication: a systematic review. Ann Intern Med. 2014;161:270–280. [DOI] [PubMed] [Google Scholar]
- 8. Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27:696–713. [DOI] [PubMed] [Google Scholar]
- 9. Ha Dinh TT, Bonner A, Clark R, Ramsbotham J, Hines S. The effectiveness of the teach‐back method on adherence and self‐management in health education for people with chronic disease: a systematic review. JBI Database System Rev Implement Rep. 2016;14:210–247. [DOI] [PubMed] [Google Scholar]
- 10. Bodenheimer T. Teach‐Back: a simple technique to enhance patients' understanding. Fam Pract Manag. 2018;25:20–22. [PubMed] [Google Scholar]
- 11. Centrella‐Nigro AM, Alexander C. Using the teach‐back method in patient education to improve patient satisfaction. J Contin Educ Nurs. 2017;48:47–52. [DOI] [PubMed] [Google Scholar]
- 12. Yi H, Xiao T, Thomas PS, et al. Barriers and facilitators to patient‐provider communication when discussing breast cancer risk to aid in the development of decision support tools. AMIA Annu Symp Proc. 2015;2015:1352–1360. [PMC free article] [PubMed] [Google Scholar]
- 13. Samson P, Waters EA, Meyers B, Politi MC. Shared decision making and effective risk communication in the high‐risk patient with operable stage I non‐small cell lung cancer. Ann Thorac Surg. 2016;101:2049–2052. [DOI] [PubMed] [Google Scholar]
- 14. Klein KA, Watson L, Ash JS, Eden KB. Evaluation of risk communication in a mammography patient decision aid. Patient Educ Couns. 2016;99:1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yen PH, Leasure AR. Use and effectiveness of the teach‐back method in patient education and health outcomes. Fed Pract. 2019;36:284–289. [PMC free article] [PubMed] [Google Scholar]
- 16. Yeh MY, Wu SC, Tung TH. The relation between patient education, patient empowerment and patient satisfaction: a cross‐sectional‐comparison study. Appl Nurs Res. 2018;39:11–17. [DOI] [PubMed] [Google Scholar]
- 17. Hillen MA, van Vliet LM, de Haes HC, Smets EM. Developing and administering scripted video vignettes for experimental research of patient‐provider communication. Patient Educ Couns. 2013;91:295–309. [DOI] [PubMed] [Google Scholar]
- 18. van Vliet LM, Hillen MA, van der Wall E, Plum N, Bensing JM. How to create and administer scripted video‐vignettes in an experimental study on disclosure of a palliative breast cancer diagnosis. Patient Educ Couns. 2013;91:56–64. [DOI] [PubMed] [Google Scholar]
- 19. van Vliet LM, van der Wall E, Albada A, Spreeuwenberg PM, Verheul W, Bensing JM. The validity of using analogue patients in practitioner‐patient communication research: systematic review and meta‐analysis. J Gen Intern Med. 2012;27:1528–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blanch‐Hartigan D, Hall JA, Krupat E, Irish JT. Can naive viewers put themselves in the patients' shoes? reliability and validity of the analogue patient methodology. Med Care. 2013;51:16–21. [DOI] [PubMed] [Google Scholar]
- 21. Zwan MD, van der Flier WM, Cleutjens S, et al. Dutch Brain Research Registry for study participant recruitment: design and first results. Alzheimers Dement (N Y). 2021;7:e12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elo S, Kyngas H. The qualitative content analysis process. J Adv Nurs. 2008;62:107–115. [DOI] [PubMed] [Google Scholar]
- 24. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288. [DOI] [PubMed] [Google Scholar]
- 25. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 26. Marteau TM, Bekker H. The development of a six‐item short‐form of the state scale of the Spielberger State‐Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31:301–306. [DOI] [PubMed] [Google Scholar]
- 27. van der Bij AK, de Weerd S, Cikot RJ, Steegers EA, Braspenning JC. Validation of the dutch short form of the state scale of the Spielberger State‐Trait Anxiety Inventory: considerations for usage in screening outcomes. Community Genet. 2003;6:84–87. [DOI] [PubMed] [Google Scholar]
- 28. Mishel MH, Braden CJ. Finding meaning: antecedents of uncertainty in illness. Nurs Res. 1988;37:98–127. [PubMed] [Google Scholar]
- 29. Zandbelt LC, Smets EMA, Oort FJ, Godfried MH, de Haes HCJM. Satisfaction with the outpatient encounter a comparison of patients’ and physicians’ views. J Gen Intern Med. 2004;19:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blanchard CG, Ruckdeschel JC, Fletcher BA, Blanchard EB. The impact of oncologists' behaviors on patient satisfaction with morning rounds. Cancer. 1986;58:387–393. [DOI] [PubMed] [Google Scholar]
- 31. Aalfs CM, Oort FJ, de Haes JCJM, Leschot NJ, Smets EMA. A comparison of counselee and counselor satisfaction in reproductive genetic counseling. Clin Genet. 2007;72:74–82. [DOI] [PubMed] [Google Scholar]
- 32. Hillen MA, Postma RM, Verdam MG, Smets EM. Development and validation of an abbreviated version of the Trust in Oncologist Scale‐the Trust in Oncologist Scale‐short form (TiOS‐SF). Support Care Cancer. 2017;25:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arraras JI, Greimel E, Sezer O, et al. An international validation study of the EORTC QLQ‐INFO25 questionnaire: an instrument to assess the information given to cancer patients. Eur J Cancer. 2010;46:2726–2738. [DOI] [PubMed] [Google Scholar]
- 34. Visser LN, Hillen MA, Verdam MG, Bol N, de Haes HC, Smets EM. Assessing engagement while viewing video vignettes; validation of the Video Engagement Scale (VES). Patient Educ Couns. 2016;99:227–235. [DOI] [PubMed] [Google Scholar]
- 35. Kunneman M, Smets EMA, Bouwman FH, et al. Clinicians' views on conversations and shared decision making in diagnostic testing for Alzheimer's disease: the ABIDE project. Alzheimers Dement (N Y). 2017;3:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mishel MH, Reconceptualization of the uncertainty in illness theory. 1990. [DOI] [PubMed]
- 37. Hillen MA, Gutheil CM, Smets EMA, et al. The evolution of uncertainty in second opinions about prostate cancer treatment. Health Expect. 2017;20:1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson LA, Day KL, Beard RL, Reed PS, Wu B. The public's perceptions about cognitive health and Alzheimer's disease among the U.S. population: a national review. Gerontologist. 2009;49(Suppl 1):S3–11. [DOI] [PubMed] [Google Scholar]
- 39. Schwabe L, Wolf OT. Learning under stress impairs memory formation. Neurobiol Learn Mem. 2010;93:183–188. [DOI] [PubMed] [Google Scholar]
- 40. Kessels RP. Patients' memory for medical information. J R Soc Med. 2003;96:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Osch M, Sep M, van Vliet LM, van Dulmen S, Bensing JM. Reducing patients' anxiety and uncertainty, and improving recall in bad news consultations. Health Psychol. 2014;33:1382–1390. [DOI] [PubMed] [Google Scholar]
- 42. Visser LNC, Tollenaar MS, de Haes H, Smets EMA. The value of physicians' affect‐oriented communication for patients' recall of information. Patient Educ Couns. 2017;100:2116–2120. [DOI] [PubMed] [Google Scholar]
- 43. Medendorp NM, Visser LNC, Hillen MA, de Haes J, Smets EMA. How oncologists' communication improves (analogue) patients' recall of information. A randomized video‐vignettes study. Patient Educ Couns. 2017;100:1338–1344. [DOI] [PubMed] [Google Scholar]
- 44. Bawden D, Holtham C, Courtney N. Perspectives on information overload. Aslib Proceedings. 1999;51:1999–1255. [Google Scholar]
- 45. Hall A, Walton G. Information overload within the health care system: a literature review. Health Info Libr J. 2004;21:102–108. [DOI] [PubMed] [Google Scholar]
- 46. Klerings I, Weinhandl AS, Thaler KJ. Information overload in healthcare: too much of a good thing? Z Evid Fortbild Qual Gesundhwes. 2015;109:285–290. [DOI] [PubMed] [Google Scholar]
- 47. Luck A, Pearson S, Maddern G, Hewett P. Effects of video information on precolonoscopy anxiety and knowledge: a randomised trial. Lancet. 1999;354:2032–2035. [DOI] [PubMed] [Google Scholar]
- 48. Arterburn DE, Westbrook EO, Bogart TA, Sepucha KR, Bock SN, Weppner WG. Randomized trial of a video‐based patient decision aid for bariatric surgery. Obesity (Silver Spring). 2011;19:1669–1675. [DOI] [PubMed] [Google Scholar]
- 49. Kakinuma A, Nagatani H, Otake H, Mizuno J, Nakata Y. The effects of short interactive animation video information on preanesthetic anxiety, knowledge, and interview time: a randomized controlled trial. Anesth Analg. 2011;112:1314–1318. [DOI] [PubMed] [Google Scholar]
- 50. Chair SY, Chau MY, Sit JW, Wong EM, Chan AW. The psychological effects of a videotape educational intervention on cardiac catheterization patients. Contemp Nurse. 2012;40:225–233. [DOI] [PubMed] [Google Scholar]
- 51. Hoppe DJ, Denkers M, Hoppe FM, Wong IH. The use of video before arthroscopic shoulder surgery to enhance patient recall and satisfaction: a randomized‐controlled study. J Shoulder Elbow Surg. 2014;23:e134–9. [DOI] [PubMed] [Google Scholar]
- 52. Sorensen K, Pelikan JM, Rothlin F, et al. Health literacy in Europe: comparative results of the European health literacy survey (HLS‐EU). Eur J Public Health. 2015;25:1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Winter M, Kam J, Nalavenkata S, et al. The use of portable video media vs standard verbal communication in the urological consent process: a multicentre, randomised controlled, crossover trial. BJU Int. 2016;118:823–828. [DOI] [PubMed] [Google Scholar]
- 54. Ahlander BM, Engvall J, Maret E, Ericsson E. Positive effect on patient experience of video information given prior to cardiovascular magnetic resonance imaging: a clinical trial. J Clin Nurs. 2018;27:1250–1261. [DOI] [PubMed] [Google Scholar]
- 55. Ahmed KJ, Pilling JD, Ahmed K, Buchan J. Effect of a patient‐information video on the preoperative anxiety levels of cataract surgery patients. J Cataract Refract Surg. 2019;45:475–479. [DOI] [PubMed] [Google Scholar]
- 56. Purcell‐Jones JMA, Haasbroek M, Van der Westhuizen JL, Dyer RA, Lombard CJ, Duys RA. Overcoming language barriers using an information video on spinal anesthesia for cesarean delivery: implementation and impact on maternal anxiety. Anesth Analg. 2019;129:1137–1143. [DOI] [PubMed] [Google Scholar]
- 57. Soydas Yesilyurt D, Yildiz Findik U. Effect of preoperative video information on anxiety and satisfaction in patients undergoing abdominal surgery. Comput Inform Nurs. 2019;37:430–436. [DOI] [PubMed] [Google Scholar]
- 58. Riedl D, Schüßler G. The Influence of doctor‐patient communication on health outcomes: a systematic review. Z Psychosom Med Psychother. 2017;63:131–150. [DOI] [PubMed] [Google Scholar]
- 59. Chandra S, Mohammadnezhad M, Ward P. Trust and communication in a doctor‐ patient relationship: a literature review. J Health Commun. 2018;3(3:36):1–6. [Google Scholar]
- 60. Birkhauer J, Gaab J, Kossowsky J, et al. Trust in the health care professional and health outcome: a meta‐analysis. PLoS One. 2017;12:e0170988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Haes H, Bensing J. Endpoints in medical communication research, proposing a framework of functions and outcomes. Patient Educ Couns. 2009;74:287–294. [DOI] [PubMed] [Google Scholar]
- 62. Bartlett EE, Grayson M, Barker R, Levine DM, Golden A, Libber S. The effects of physician communications skills on patient satisfaction; recall, and adherence. J Chronic Dis. 1984;37:755–764. [DOI] [PubMed] [Google Scholar]
- 63. Kessels RPC. Patients’ memory for medical information. J Roy Soc Med. 2003;96:219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Linn AJ, van Dijk L, Smit EG, Jansen J, van Weert JC. May you never forget what is worth remembering: the relation between recall of medical information and medication adherence in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:e543–50. [DOI] [PubMed] [Google Scholar]
- 65. Visser LNC, Kunneman M, Murugesu L, et al. Clinician‐patient communication during the diagnostic workup: the ABIDE project. Alzheimers Dement (Amst). 2019;11:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van Maurik IS, Visser LN, Pel‐Littel RE, et al. Development and usability of ADappt: web‐Based tool to support clinicians, patients, and caregivers in the diagnosis of mild cognitive impairment and Alzheimer disease. JMIR Form Res. 2019;3:e13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


