Abstract
Background/Aims
Hyaluronan (HA) oligosaccharides are involved in several biological processes, primarily collagen remodeling and wound healing. Collagen remodeling is retarded in aging skin and causes wrinkles. The aim of this study was to evaluate the effect of 2‐kDa HA oligosaccharides (HA2k) on wrinkles by permeation through the stratum corneum and promotion of collagen remodeling.
Methods
A 3D skin model and excised human skin were used to evaluate the permeation of fluorescein‐labeled HA2k. The effect of HA2k on collagen metabolism was evaluated by measuring the protein level of type 1 pro‐collagen (COL1A1) and matrix metalloproteinase‐1 (MMP‐1) in the 3D skin model. 0.1% HA2k solution and vehicle control was applied to the human forearm for 8 weeks to evaluate dermal collagen density. To evaluate the effect of HA2k on depth of facial wrinkles, a randomized controlled trial was conducted with 0.1% HA2k lotion and vehicle lotion for 8 weeks.
Results
HA2k was confirmed to permeate through the stratum corneum by fluorescent microscopy. Both COL1A1 and MMP‐1 were upregulated by HA2k application in a 3D skin model culture. The collagen density was higher for the HA2k‐treated forearm than for the vehicle control‐treated forearm after 4 weeks. The maximum wrinkle depths in the nasolabial fold and crow's feet area were significantly shallower in the HA2k lotion group than in the control group.
Conclusion
HA2k permeated the stratum corneum, activated collagen synthesis and degradation simultaneously, and ameliorated wrinkles.
Keywords: anti‐aging, cell culture, collagen remodeling, human study, hyaluronan
1. INTRODUCTION
The extracellular matrix (ECM) is a composite of macromolecules, including proteins and glycosaminoglycans, that provides mechanical support to surrounding cells. In dermal tissue, the ECM contributes to the physical properties of skin. For instance, collagen, the primary component of dermal ECM, contributes to the integrity, elasticity, and smoothness of skin. 1 Physiologically, skin collagen is maintained by degradation of old collagen and re‐synthesis of new collagen in a process called collagen remodeling. 2 However, the cycle of collagen remodeling can be retarded in aging skin, resulting in wrinkling, sagging, and decreased elasticity. 3 These problems are significantly related to a person's higher perceived age than actual age. Therefore, collagen remodeling is a promising target for anti‐aging. An approach that targets ECM potentially could be better for managing these problems because superficial approaches do not affect ECM. Micro‐needling is a way that targets ECM to promote dermal collagen remodeling; however, it is a relatively invasive method.
Hyaluronan (HA), a biologically derived polysaccharide, is also a component of ECM. HA was first discovered in the vitreous body of the eye and later in the synovial fluid, umbilical cord, and skin. The molecular weight of HA differs according to the type of tissue and its biological state, ranging from 1 kDa to 3000 kDa. 4 HA in the skin generally exists in a high‐molecular‐weight state, and owing to its high viscoelasticity and water‐holding ability, HA functions as a cushion, lubricant, and moisturizer. High‐molecular‐weight HA is, therefore, used in the field of cosmetics to maintain the water content of the stratum corneum. Since macromolecules have very limited permeability through the skin, there have been some attempts to improve HA permeability by degrading HA into small fragments, which have been confirmed to permeate through the stratum corneum. 5
In addition to physical function, the biological function of HA has been a recent topic of research. Some noteworthy reports have been published regarding HA's biological function as a component of the stem cell niche 6 and the extraordinary longevity of naked mole rats. The molecular weight of HA needs to be considered when its biological functions are discussed. HA in the skin generally exists in a high‐molecular‐weight state and reportedly maintains tissue stability. In contrast, low‐molecular‐weight HA activates several biological processes. 7 In previous reports, HA tetrasaccharide upregulated synthesis of HA and type I collagen in co‐cultured keratinocytes and fibroblasts, 8 induced differentiation markers in keratinocytes, 9 and ameliorated the damage in an ultraviolet‐A‐irradiated mouse. 5 In particular, oligosaccharides below 10 kDa primarily promote wound‐healing processes, such as ECM synthesis, 9 angiogenesis, cellular migration, and proliferation. Wound healing and collagen remodeling are closely related, and a 12‐mer (i.e., approximately 2 kDa) of HA upregulates type I collagen and matrix metalloproteinase 1 (MMP‐1) simultaneously in cultured fibroblasts, but the effect on humans remains to be clarified.
There have been some reports of HA cosmetics that showed anti‐wrinkle effects. A skin cream of 50‐kDa HA ameliorated the wrinkle depth of female subjects aged 30–60 years in a randomized half‐side comparison trial. 10 An open‐labeled study showed that micronized‐HA cream was topically applied to 35–65‐year‐old subjects, which resulted in improvement of questionnaire scores on wrinkle depth. 11 In these studies, the high permeability of low‐molecular‐weight HA was suggested to be a mechanism of action, although there had been no data in human skin. In addition, the effect of HA fragments on collagen density in humans remains to be clarified.
We hypothesized that HA oligosaccharides permeate through the stratum corneum, promote collagen remodeling, and improve wrinkles as an anti‐aging effect. This study aimed to evaluate the effects of 2‐kDa HA (HA2k) on collagen remodeling and skin conditions via in vitro and in vivo studies. First, permeation of HA2k was assessed in a reconstituted skin model and human excised skin. Subsequently, the effects on collagen remodeling were evaluated in vitro and in human subjects. Finally, a randomized half‐side comparison of HA2k‐containing skin lotion and control lotion was performed among 21 subjects to assess the effects on skin wrinkles.
2. MATERIALS AND METHODS
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Kewpie Corp. and the DERMAPRO Ltd. Institutional Review Board, with approval numbers 17–4 and 1‐220 777‐A‐N‐02‐DICN18228, respectively. This study was conducted with the voluntary consent of the subjects.
2.1. Reagents
HAbooster™, a cosmetic‐grade HA2k reagent, was obtained from Kewpie Corp. (Japan).
2.2. Skin permeation of HA2k in three‐dimensional cultured human epidermis and excised human skin
Permeation of HA2k was evaluated by using a Franz‐type diffusion cell, according to a previous report. 12 Fluorescein‐labeled (Fl‐) HA samples were prepared at <1.25 mg/ml HA in water and dimethyl sulfoxide mixed at a ratio of 2:1 (v:v). Next, 50 mg/ml each of 5‐aminofluorescein, cyclohexyl isocyanide, and acetaldehyde were added to bind fluorescein to HA. After 5 h of reaction at room temperature in the dark, the sample was purified by gel filtration chromatography using a PD Midi Trap G‐10 (GE Healthcare,) with an exclusion lower limit of 700 M r. The obtained FL‐HA solution was lyophilized and used in the following experiments. For evaluation with reconstituted skin by cell culture, a 3D skin model EPI‐606X (Mat Tek,), which was confirmed to retain barrier function, was fixed in a Franz‐type diffusion cell. The three‐dimensional (3D) skin model was precultured for 16–18 h at 37°C, 5% CO2 in the medium provided by the supplier. After the equilibration process, the medium was changed for testing. A 1% solution of Fl‐HA samples of 2 or 8 kDa were applied to the upper compartment and then incubated for 5 h. After washing four times with phosphate‐buffered saline, the specimen was embedded in OCT compound (Sakura Finetek,) and frozen by using dry ice/acetone for the preparation of tissue section. For evaluation on human skin, dermatomed abdominal skin from a 34‐year‐old Caucasian woman (Biopredic International, France) was fixed in a Franz‐type diffusion cell. A 0.1% Fl‐HA solution (molecular weight, 2 or 1000 kDa) was applied to the human skin. After incubation at 32°C for 24 h, the tissue was subjected to cryosection preparation as described above. The tissue slides were observed under a fluorescent microscope.
2.3. Evaluation of collagen metabolism in a 3D skin model and excised human skin
A 3D skin model was used to evaluate the effects of HA2k on collagen metabolism. 13 In brief, a full‐thickness model (EFT‐412, Mat Tek) comprising keratinocytes and fibroblasts was cultured in an 8 μg/ml HA2k‐containing medium. After 48 hours, the conditioned medium was collected, and enzyme‐linked immunosorbent assay (ELISA) the levels of type 1 pro‐collagen (COL1A1) and MMP‐1 were measured. The tissue was processed for immunohistological analysis. Collagen in tissues was assessed by using COL1A1 immunohistochemical staining on the tissue slides of formalin‐fixed paraffin‐embedded sections. The sections were treated with proteinase K for 10 min at 37°C to retrieve antigen. After blocking, the anti‐COL1A1 monoclonal antibodies (Abcam,) were incubated at 4°C overnight. The slides were then incubated with Alexa Fluor® 488 Goat Anti‐Rabbit IgG (Abcam) at room temperature for 2 h. The nucleus was counterstained with propidium iodide. The sections were observed under a fluorescent microscope.
2.4. Evaluation of skin collagen density, moisture, and tomography in human subjects
HA2k was topically applied to human skin to evaluate its function as an active ingredient. Eight subjects (two women and six men; age, 26–39 years) voluntarily enrolled in the study. Their forearms were divided into two areas measuring 2 cm × 2 cm for the samples to be applied twice a day. The 0.1% of HA2k solution and vehicle control were applied separately to the two areas. At 0, 4, and 8 weeks, the collagen score, skin moisture, and skin roughness were evaluated. The subjects were acclimated under a controlled atmosphere at 23°C ± 1°C and a relative humidity of 55% ± 2% for 20 min. This acclimation period was followed by measurement.
2.5. Evaluation of collagen score
Ultrasound was used to measure the skin collagen density (DermaLab, Cortex Technology, Denmark). 14 The probe was applied on the HA2k lotion and vehicle control sites to measure the collagen scores. A total of five points, comprising the four corners and center of each site, were measured, and the average value was calculated.
2.6. Evaluation of skin moisture
Skin moisture was measured by using the impedance method (SKICON, Yayoi,). 15 The probe was applied on the evaluation site to measure the skin moisture. A total of five points at the four corners and center were measured. The average value was calculated.
2.7. Evaluation of skin roughness
The skin roughness was measured by visual analysis (VisioScan, Courage + Khazaka Electronic, Germany). 16 Images were acquired at the four corners and analyzed by the software. The average value was calculated.
2.8. Evaluation of wrinkle, sagging, and elasticity of the skin in human subjects
Given that dermal collagen is reportedly associated with wrinkling, sagging, and elasticity of the skin, application of HA2K in cosmetic products was evaluated. Twenty‐one subjects were enrolled according to the following criteria: healthy females with mild‐to‐moderate wrinkles by visual and photo assessment ranging from wrinkle grades 1–3 according to the Japanese Cosmetic Science Society wrinkle grading guideline, having nasolabial fold, neither pregnant nor nursing, and not having sensitivity nor hypersensitivity skin. During this testing period, the subjects did not use any cosmetic products, beauty tools, or skin care procedures besides the test article. The sample products provided as lotion were applied twice daily (morning and evening). Formulations of the lotions are shown in Table 1. After washing the face, the lotions were applied, followed by a designated milky lotion and cream, if necessary. HA2k lotion was applied on one side, and the control product was applied on the other side. The subjects were examined by a 3D image analyzing system to assess crow's feet, nasolabial fold, skin sagging, and elasticity at baseline (0 week), 4 weeks, and 8 weeks after treatment. All measurements were performed under a given relative temperature (20°C–24°C) and humidity (45%–55%).
TABLE 1.
Skin lotion formulation used in the half‐side comparison study
| Ingredient | Formulation (%) | |
|---|---|---|
| Control | HA2k | |
| Glycerin | 3 | 3 |
| Methylparaben | 0.1 | 0.1 |
| Phenoxyethanol | 0.2 | 0.2 |
| Alcohol | 4.5 | 4.5 |
| PEG*‐32 | 2 | 2 |
| 1, 3 butylene glycol | 2 | 2 |
| Citric acid | 0.01 | 0.01 |
| Sodium citrate | 0.09 | 0.09 |
| Disodium EDTA** | 0.1 | 0.1 |
| Hydrolyzed sodium hyaluronate (HA2k) | — | 0.1 |
| Water | 88 | 87.9 |
| Total | 100 | |
Abbreviations: *PEG, polyethylene glycol; **EDTA, ethylenediaminetetraacetic acid.
2.9. Measurement of wrinkle parameters
The wrinkle parameters of the crow's feet and nasolabial fold were evaluated by visual analysis (PRIMOS® Premium and Lite, GFMesstechnik,). 17 The image was analyzed in the same area at baseline, 4, and 8 weeks after treatment by using software (Primos 5.8 E ver.).
2.10. Measurement of skin elasticity
Skin elasticity was measured by the suction method (Cutometer® MPA580, Courage + Khazaka electronic,) according to a previous report. 18 In brief, skin was drawn into the aperture of the probe at 450 mbar for 2 seconds. Subsequently, the negative pressure was switched off for 2 s and the skin allowed to return to its original shape. Three repetitions in one measuring cycle were performed. The skin elasticity on the cheek was analyzed by R2 parameters at baseline, 4, and 8 weeks after treatment.
2.11. Measurement of skin sagging
Skin sagging on the cheek was evaluated by using the Moire topography method (F‐ray®, Beyoung,), 19 which is a three‐dimensional morphometric method in which contour maps are produced from the overlapping interference fringes created when an object is illuminated by coherent light. The Moire pattern on the cheek was taken, and the angle produced by drawing two lines at the end of the nose was analyzed by using software (Image‐Pro Plus®, MediaCybernetics,) at baseline, 4, and 8 weeks after treatment.
2.12. Statistical analysis
Statistical analysis was performed by using the SPSS® software program (IBM,). The Mann–Whitney U‐test was performed to evaluate the effect of HA2k on collagen remodeling in vitro. The effect of HA2k on collagen in human subjects was analyzed by using the paired t‐test. In human studies that evaluated the effect of HA2k on wrinkles, sagging, and elasticity of skin, the Shapiro–Wilks test or kurtosis and skewness was employed for normality testing. Statistical analysis of parametric variables was performed by using repeated measures ANOVA. If the value was nonparametric, all were initially compared by using the Wilcoxon signed‐rank test with Bonferroni correction. Statistical analysis for comparison between sites was performed by repeated measures ANOVA or ANCOVA using the variation value from baseline to 4 and 8 weeks after treatment. A p value of <0.05 was considered to be indicative of statistical significance. Change from baseline (%) was defined as follows: (Baseline − After treatment)/Baseline × 100.
3. RESULTS
3.1. Skin permeation of HA2k
In a 3D‐skin model, fluorescein‐labeled HA2k passed through the stratum corneum (Figure 1), whereas the 8‐kDa HA did not. In human skin, fluorescein‐labeled HA2k penetrated to the dermis. The 1000‐kDa HA did not pass through the stratum corneum. These results indicate that compared with larger molecular weight HA, HA2k had high dermal permeability.
FIGURE 1.
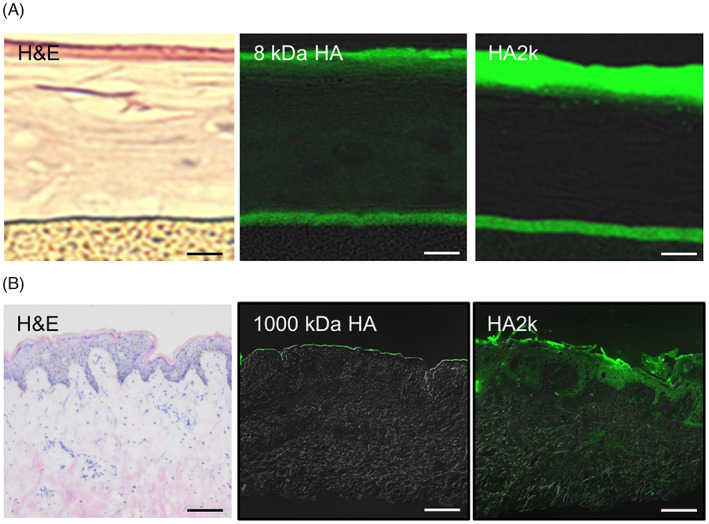
Transdermal penetration of HA2k in three‐dimensional cultured human epidermis and excised human skin.
In the upper panel (A), 1% fluorescein‐labeled HA2k and 8‐kDa HA were applied to a 3D skin model. The HA2k passed through the stratum corneum, whereas the 8‐kDa HA did not. Magnification, x200; scale bars, 50 μm. In the lower panel (B), 0.1% fluorescein‐labeled HA2k and 1000‐kDa HA were applied to a human skin sample. The HA2k passed through the stratum corneum, whereas the 1000‐kDa HA did not. Magnification, x100; scale bars, 100 μm
3.2. Effect of HA2k on collagen metabolism
In a 3D‐skin model, the protein contents of both COL1A1 and MMP‐1 in the conditioned medium were upregulated by HA2k addition (Figure 2). Therefore, HA2k was assumed to simultaneously promote the production of COL1A1 and MMP‐1.
FIGURE 2.
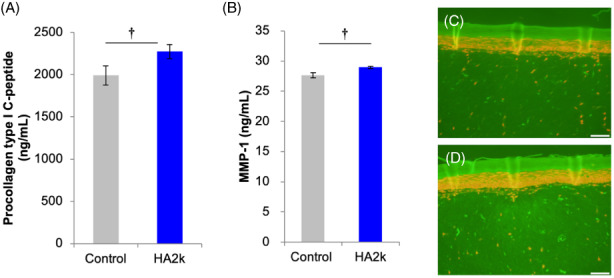
Effect of HA2k on collagen metabolism in a three‐dimensional cultured human dermal–epidermal model.
Addition of an HA2k solution at 8 μg/ml to a 3D skin model to evaluate COL1A1 (A) and MMP‐1 (B) by performing an ELISA. Immunohistochemistry image of COL1A1, Control (C) and HA2k (D).
†Significantly different at p 〈 0.05, mean ± SD, n = 3. Magnification, x100; Scale bars, 100 μm
3.3. Effect of HA2k on collagen content and skin status in human subjects
In the human studies, dermal collagen scores were higher after 4 weeks at the site where 0.1% HA2k solution had been applied than at the site where the control solution had been applied (Figure 3). Additionally, the statuses of the skin water content and skin roughness were better after 8 weeks at the site treated with the HA2k solution than at the site treated with the control solution. Therefore, it was suggested that HA2k promotes collagen production in human skin and improves its condition.
FIGURE 3.
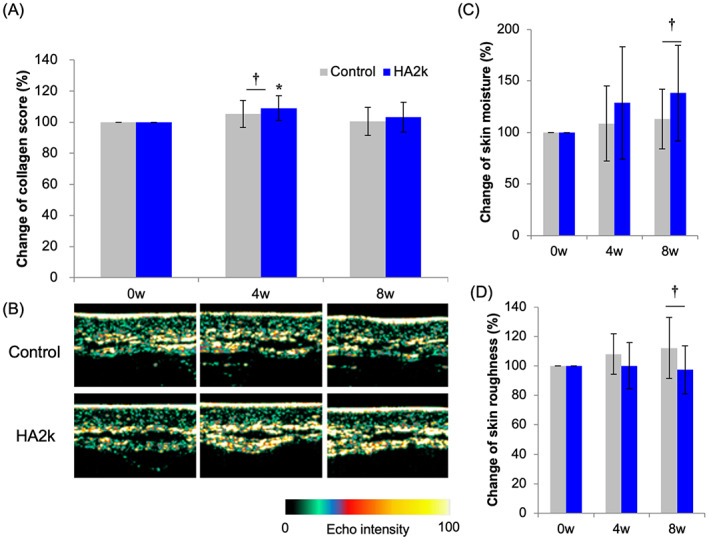
Effect of HA2k on skin collagen density, moisture, and tomography in a human forearm
A 0.1% HA2k lotion applied to a human forearm. (A) Change in the collagen score measured by an ultrasound method; (B) Visualized collagen density; (C) Change in skin moisture measured by an impedance method; and (D) Change in skin roughness measured by visual analysis. †Significantly different at p < 0.05, *significantly different compared with baseline at p < 0.05, mean ± SD, n = 8
3.4. Effect of HA2k on wrinkle, sagging, and elasticity of the skin in human subjects
In the human test, the maximum depth of crow's feet and nasolabial wrinkles were lower at 4 weeks and 8 weeks on the side treated with 0.1% HA2k lotion than on the side treated with the control lotion (Figure 4). Skin sagging was improved on the side treated with the HA2k lotion after 4 weeks than at baseline. Skin elasticity was improved after 4 and 8 weeks on the side treated with the HA2k lotion, whereas it was improved only after 8 weeks on the side treated with the control lotion. These results indicate that the HA2k lotion improved wrinkling, sagging, and elasticity of the skin.
FIGURE 4.
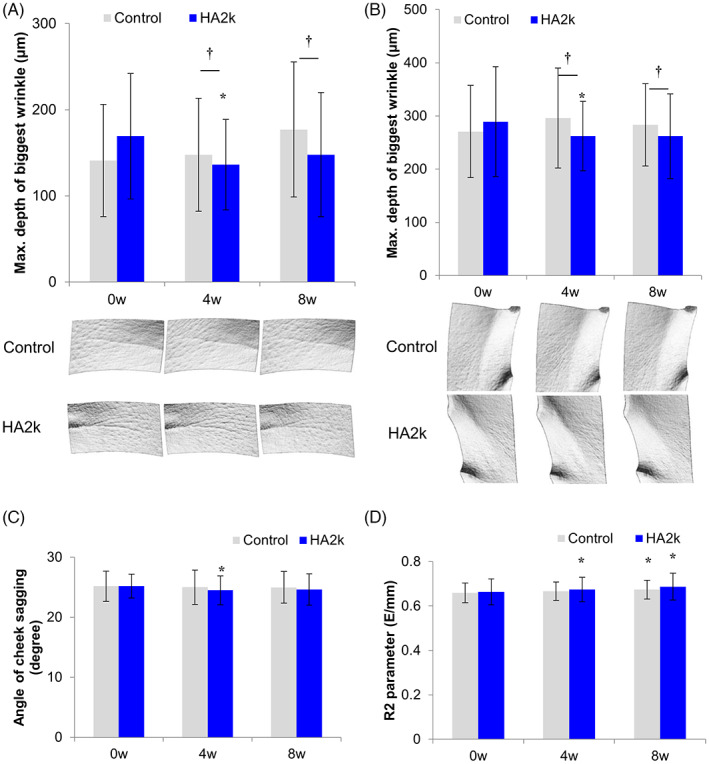
Effect of 0.1% HA2k lotion on facial wrinkles. A 0.1% HA2k lotion was applied to the faces of the human subjects. Maximum depth of the biggest wrinkle in the crow's feet facial area (A) and nasolabial fold (B) measured by visual analysis. (C) Skin sagging angle measured by the Moire topography method. (D) Skin elasticity based on R2 parameter measured by a suction method. †Significantly different at p < 0.05, *significantly different compared with baseline at p < 0.05, mean ± SD, n = 21
4. DISCUSSION
It has been reported that the nasolabial fold is one of the strongest factors that affect the gap between a person's actual age and perceived age. Filler injection is a common way to improve the nasolabial fold; however, the effect and safety depend on the technique of physicians. Some severe side effects of injection caused by vascular occlusion leading to skin necrosis and blindness have been reported. 20 HA2k is a safer and less invasive option to improve the nasolabial fold. Furthermore, HA2k was tested as a formulated lotion in this study and was supposed to be a more practical active cosmetic ingredient (ACI).
The nasolabial fold is caused by aging in the dermis, and it is difficult to improve by approaching only the upper layer of the epidermis. To deliver ACIs to deeper layers, such as the epidermis and dermis, it is necessary to permeate through the stratum corneum. Previously, <300‐kDa HAs have been shown to permeate through the stratum corneum of excised human skin. 21 It is, therefore, reasonable that HA2k permeates through the stratum corneum. Interestingly, a 1200‐kDa HA has been reported to permeate through the stratum corneum of excised skin when polyion complex particles were constructed with a specific polycation. 22 It is suggested that higher transdermal absorption of HA2k would be achieved by optimizing the formulation.
Some reports have stated that ACIs upregulate collagen synthesis. We previously reported on such ACIs, including HA tetrasaccharides, 8 soybean peptides, and collagen peptides. HA2k is unique because it upregulates collagen synthesis and degradation simultaneously. There is a need to clarify the mechanism of upregulation of COL1A1 and MMP‐1 in future studies.
Low‐molecular‐weight HA has been reported to induce inflammation in the skin 23 ; however, the mechanism of action remains controversial. Another study using a reconstituted epidermis reported that a low‐molecular‐weight HA was inflammatory. 24 In our human study, no side effects, including inflammatory symptoms, were observed. It is possible that low‐molecular‐weight HAs induce inflammatory signaling only in vitro, without a dermis. Under more complex conditions, such as those of human skin, crosstalk occurs among various cells of tissues, and the inflammatory signaling is canceled. It is noteworthy that a recent study showed that endogenous HA fragmentation induced inflammatory responses, whereas exogenous low‐molecular‐weight HAs (2800–60 000 Da) did not. 25
The concentration of HA2k used in this human study was 0.1%, but its optimum dose remains to be clarified. HA2k can be more widely used if lower doses are effective. In addition, combined with higher‐molecular‐weight HAs would enable development of more effective cosmetics for anti‐aging because larger HAs are better moisturizers. 4 HA2k is still a relatively cost‐effective material for improving wrinkles since it is not administered intravenously by a physician.
5. CONCLUSION
In this study, we investigated the effect of HA2k on improving skin wrinkles by topical application to human skin. The nasolabial fold and crow's feet were significantly improved by a 0.1% HA2k lotion. The first step in the proposed mechanism is permeation of HA2k through the stratum corneum, which we confirmed by performing a permeation experiment in a 3D skin model and human excised skin. The second step of the mechanism is activation of collagen remodeling by the HA2k, which we demonstrated by performing an ELISA on COL1A1 and MMP‐1 in a 3D skin model and by ultrasound analysis of collagen density in a pilot study in human subjects. Taken together, HA2k is a promising ingredient for anti‐aging cosmetics.
AUTHOR CONTRIBUTIONS
Y.A. and Y.T. designed research. Y.A., H.K., and M.K. performed experiments. Y.A. and S.S. analyzed data, and S.S wrote the manuscript supervised by T.Y. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
Y.T. and M.K declare that they have no competing interests.
Y.A., S.S., and H.K. are employee of Kewpie Corporation, supplier of HA.
ETHICAL APPROVAL
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Kewpie Corp. and the DERMAPRO Ltd. Institutional Review Board, with approval numbers 17‐4 and 1‐220777‐A‐N‐02‐DICN18228, respectively.
ACKNOWLEDGMENT
Scientific writing assistance was provided by Crimson Interactive Pvt. Ltd.
Abe Y, Seino S, Kurihara H, Kage M, Tokudome Y. 2‐kDa hyaluronan ameliorates human facial wrinkles through increased dermal collagen density related to promotion of collagen remodeling. J Cosmet Dermatol. 2023;22:320‐327. doi: 10.1111/jocd.15097
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Arseni L, Lombardi A, Orioli D. From structure to phenotype: impact of collagen alterations on human health. Int J Mol Sci. 2018;19:1407. doi: 10.3390/ijms19051407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Page‐McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee DH, Oh JH, Chung JH. Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci. 2016;83:174‐181. [DOI] [PubMed] [Google Scholar]
- 4. Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27‐33. doi: 10.1046/j.1365-2796.1997.00170.x [DOI] [PubMed] [Google Scholar]
- 5. Kage M, Tokudome Y, Hashimoto F. Permeation of hyaluronan tetrasaccharides through hairless mouse skin: an in vitro and in vivo study. Arch Dermatol Res. 2013;305:69‐77. doi: 10.1007/s00403-012-1252-2 [DOI] [PubMed] [Google Scholar]
- 6. Gesteira TF, Sun M, Coulson‐Thomas YM, et al. Hyaluronan rich microenvironment in the limbal stem cell niche regulates limbal stem cell differentiation. Invest Ophthalmol Vis Sci. 2017;58:4407‐4421. doi: 10.1167/iovs.17-22326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kavasi RM, Berdiaki A, Spyridaki I, et al. HA metabolism in skin homeostasis and inflammatory disease. Food Chem Toxicol. 2017;101:128‐138. doi: 10.1016/j.fct.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 8. Kage M, Tokudome Y, Matsunaga Y, Hariya T, Hashimoto F. Expression of hyaluronan synthase and collagen type I mRNA by hyaluronan tetrasaccharides in normal human dermal fibroblasts. J Japanese Cosmet Sci Soc. 2013;37:1‐5. [Google Scholar]
- 9. Kage M, Tokudome Y, Matsunaga Y, Hariya T, Hashimoto F. Effect of hyaluronan tetrasaccharides on epidermal differentiation in normal human epidermal keratinocytes. Int J Cosmet Sci. 2014;36:109‐115. doi: 10.1111/ics.12105 [DOI] [PubMed] [Google Scholar]
- 10. Pavicic T, Gauglitz GG, Lersch P, et al. Efficacy of cream‐based novel formulations of hyaluronic acid of different molecular weights in anti‐wrinkle treatment. J Drugs Dermatol. 2011;10:990‐1000. [PubMed] [Google Scholar]
- 11. Lubart R, Yariv I, Fixler D, Lipovsky A. Topical hyaluronic acid facial cream with new micronized molecule technology effectively penetrates and improves facial skin quality: results from in‐vitro, ex‐vivo, and in‐vivo (open‐label) studies. J Clin Aesthet Dermatol. 2019;12:39‐44. [PMC free article] [PubMed] [Google Scholar]
- 12. Kano S, Todo H, Sugie K, et al. Utilization of reconstructed cultured human skin models as an alternative skin for permeation studies of chemical compounds. Altern Anim Test Exp. 2010;15:61‐70. [Google Scholar]
- 13. McDaniel DH, Hamzavi IH, Zeichner JA, et al. Total defense + repair: a novel concept in solar protection and skin rejuvenation. J Drugs Dermatol. 2015;14:s3‐s11. [PubMed] [Google Scholar]
- 14. Vitral GLN, Aguiar RAPL, de Souza IMF, Rego MAS, Guimarães RN, Reis ZSN. Skin thickness as a potential marker of gestational age at birth despite different fetal growth profiles: a feasibility study. PLoS One. 2018;13:e0196542. doi: 10.1371/journal.pone.0196542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarys P, Clijsen R, Taeymans J, Barel AO. Hydration measurements of the stratum corneum: comparison between the capacitance method (digital version of the Corneometer CM 825) and the impedance method (Skicon‐200EX). Skin Res Technol. 2012;18:316‐323. doi: 10.1111/j.1600-0846.2011.00573.x [DOI] [PubMed] [Google Scholar]
- 16. Li X, Yuan C, Xing L, Humbert P. Topographical diversity of common skin microflora and its association with skin environment type: an observational study in Chinese women. Sci Rep. 2017;7:18046. doi: 10.1038/s41598-017-18181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roques C, Téot L, Frasson N, Meaume S. PRIMOS: an optical system that produces three‐dimensional measurements of skin surfaces. J Wound Care. 2003;12:362‐364. doi: 10.12968/jowc.2003.12.9.26539 [DOI] [PubMed] [Google Scholar]
- 18. Matsunaga Y, Fujiwara S, Mori Y, et al. Development of self‐dissolving microneedles consisting hyaluronic acid as an anti‐wrinkle treatment. IFSCC Magazine. 2012;15:73‐78. [Google Scholar]
- 19. Borkow G, Elías AC. Facial skin lifting and brightening following sleep on copper oxide containing pillowcases. Cosmetics. 2016;3:24. doi: 10.3390/cosmetics3030024 [DOI] [Google Scholar]
- 20. Sito G, Manzoni V, Sommariva R. Vascular complications after facial filler injection: a literature review and meta‐analysis. J Clin Aesthet Dermatol. 2019;12:E65‐E72. [PMC free article] [PubMed] [Google Scholar]
- 21. Essendoubi M, Gobinet C, Reynaud R, Angiboust JF, Manfait M, Piot O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Skin Res Technol. 2016;22:55‐62. doi: 10.1111/srt.12228 [DOI] [PubMed] [Google Scholar]
- 22. Tokudome Y, Komi T, Omata A, Sekita M. A new strategy for the passive skin delivery of nanoparticulate, high molecular weight hyaluronic acid prepared by a polyion complex method. Sci Rep. 2018;8:2336. doi: 10.1038/s41598-018-20805-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221‐264. doi: 10.1152/physrev.00052.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farwick M, Gauglitz G, Pavicic T, et al. Fifty‐kDa hyaluronic acid upregulates some epidermal genes without changing TNF‐α expression in reconstituted epidermis. Skin Pharmacol Physiol. 2011;24:210‐217. doi: 10.1159/000324296 [DOI] [PubMed] [Google Scholar]
- 25. Takabe P, Kärnä R, Rauhala L, Tammi M, Tammi R, Pasonen‐Seppänen S. Melanocyte hyaluronan coat fragmentation enhances the UVB‐induced TLR‐4 receptor signaling and expression of proinflammatory mediators IL6, IL8, CXCL1, and CXCL10 via NF‐kappaB activation. J Invest Dermatol. 2019;139:1993‐2003. doi: 10.1016/j.jid.2019.03.1135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


