Gadolinium retention in the brains of patients with MS is not associated with long-term motor or cognitive outcomes.
Abstract
BACKGROUND AND PURPOSE:
The long-term impact of gadolinium retention in the dentate nuclei of patients undergoing administration of seriate gadolinium-based contrast agents is still widely unexplored. The aim of this study was to evaluate the impact of gadolinium retention on motor and cognitive disability in patients with MS during long-term follow-up.
MATERIALS AND METHODS:
In this retrospective study, clinical data were obtained from patients with MS followed in a single center from 2013 to 2022 at different time points. These included the Expanded Disability Status Scale score to evaluate motor impairment and the Brief International Cognitive Assessment for MS battery to investigate cognitive performances and their respective changes with time. The association with qualitative and quantitative MR imaging signs of gadolinium retention (namely, the presence of dentate nuclei T1-weighted hyperintensity and changes in longitudinal relaxation R1 maps, respectively) was probed using different General Linear Models and regression analyses.
RESULTS:
No significant differences in motor or cognitive symptoms emerged between patients showing dentate nuclei hyperintensity and those without visible changes on T1WIs (P = .14 and 0.92, respectively). When we tested possible relationships between quantitative dentate nuclei R1 values and both motor and cognitive symptoms, separately, the regression models including demographic, clinical, and MR imaging features explained 40.5% and 16.5% of the variance, respectively, without any significant effect of dentate nuclei R1 values (P = .21 and 0.30, respectively).
CONCLUSIONS:
Our findings suggest that gadolinium retention in the brains of patients with MS is not associated with long-term motor or cognitive outcomes.
The role of gadolinium-based contrast agents (GBCAs) in neuroradiologic clinical practice is unquestionable. Nevertheless, during the past years, possible consequences of their repeat administration have been reported.1 Since 2014, an increased interest in brain gadolinium (Gd) retention has emerged, especially for those patients undergoing multiple GBCA administrations during their life.2 This scenario applies to patients with malignancies3 as well as inflammatory conditions such as MS for whom contrast administration is recommended at the time of diagnosis4 and often repeated during clinical relapses and to monitor the effectiveness of disease-modifying therapy (DMT) and subclinical disease activity (particularly when previous studies for comparison are not available) or when opportunistic CNS infections are suspected.5
From a radiologic standpoint, brain Gd retention results in the development of a T1WI hyperintensity detectable on conventional imaging at the level of deep gray matter structures, with particular reference to the globus pallidus and, mostly, the dentate nuclei (DN). Several ex vivo and preclinical models have confirmed this finding, linking the development of such modifications to the number of previous GBCA administrations6 and, in particular, to linear compounds,7 to the point that the use of some Gd chelates has been restricted since 2017.8
However, while a large body of evidence supports the relationship between GBCA administration and development of T1WI hyperintensity,2,3,6,9 the clinical impact of Gd deposition is still underexplored, and available investigations provide conflicting results. Indeed, no significant association has emerged between cumulative Gd exposure and the development of parkinsonism in a population study.10 In MS, while there seems to be no association between DN hyperintensity and worsening of motor symptoms,11 Gd retention has been associated with cerebellar dysarthria and lower verbal fluency scores.12,13 So far, to the best of our knowledge, only 1 study has explored long-term clinical outcomes of Gd deposition in a small cohort of patients with MS evaluated at different time points during follow-up.12
Given this background, the aim of this study was to expand the current knowledge about the possible clinical impact of Gd accumulation in the brain, using MS as a model of a chronic condition with multiple exposures to GBCA. To accomplish this aim and investigate the presence of a delayed GBCA toxicity in patients with MS, we evaluated the long-term effects of GBCA retention on motor worsening, cognitive performance, and cognitive worsening during a 7-year follow-up period.
MATERIALS AND METHODS
Compliance with Ethical Standards
This study was approved by the local ethics committee (Carlo Romano Ethical Committee of the University of Naples “Federico II”, Approval no. 209/13) in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from each patient before enrollment.
Participants
This retrospective analysis was conducted on the same group of 74 patients with relapsing-remitting MS described in a previous work.14 Inclusion and exclusion criteria, as well as information about the number and type of previous GBCA administrations, were also reported in previously published works.11,14 All MR images used in the current analysis were acquired between 2013 and 2015. Given that these MR images were obtained before 2017, when the use of linear GBCA was limited by international agencies such as the European Medicines Agency, in some of these patients, a linear GBCA was administered (with a proportion of around 26% of linear GBCA, 45/175). Overall, a mean number of 6 GBCAs (range, 1–15) had been administered before the index MR imaging analyzed in the current work, all with the recommended standard doses.
Of 74 patients, 32 (43.2%) had concomitant comorbidities (cardiovascular comorbidities, n = 11; autoimmune comorbidities, n = 11; psychiatric comorbidities, n = 7; digestive system comorbidities, n = 6; neurologic comorbidities, n = 5; metabolic comorbidities, n = 3; respiratory comorbidities, n = 3; genitourinary comorbidities, n = 1; musculoskeletal comorbidities, n = 1).
In line with expert consensus opinions and international guidelines on the use of MR imaging for disease monitoring,15 all patients underwent a yearly brain MR imaging with Gd from the time of diagnosis onward until the recent change in monitoring recommendations.16
With reference to motor evaluation, 11 patients were lost to follow-up, leading to a final cohort of 63 subjects. All patients fulfilled the 2010 revision of the McDonald criteria at the time of MS diagnosis. The Expanded Disability Status Scale (EDSS) scores were obtained by experienced neurologists (V.B.M. and R.L, both with >25 years of experience) within 1 week from the baseline MR imaging and after a mean follow-up of 7.6 (SD, 0.6) years. Changes in the EDSS score (ΔEDSS) were calculated, in line with a previous study,11 as the subtraction of EDSS score on follow-up from the baseline EDSS score, defining motor worsening if a subject showed a ΔEDSS of ≥ 1 (for a baseline EDSS ≤ 5.5) or ≥0.5 (for a baseline EDSS > 5.5).
Although cognitive evaluations were not routinely performed at the time of the baseline MR imaging and from 2020 to 2022 due to practice modifications related to the pandemic, the Brief International Cognitive Assessment for MS (BICAMS) battery17 for most enrolled subjects (65/74, 87.8%) was collected by an experienced neuropsychologist (F.F., with >10 years of experience) after a mean follow-up of 4.6 (SD, 1.0) years and in a subset of 32 patients also after 7.5 (SD, 0.7) years from baseline MR imaging.
Briefly, the BICAMS includes the Symbol Digit Modalities Test (SDMT) to assess attention and processing speed, the California Verbal Learning Test (CVLT) to assess episodic verbal learning and memory, and the Brief Visuospatial Memory Test (BVMT) to assess visuospatial memory. Corresponding z scores were estimated according to previous works.18,19 Patients were defined as cognitively impaired if they showed at least 1 of the z score values of equal or less than –1.5.20 For the ancillary analysis, cognitive worsening with time was defined by a ΔBICAMS of equal or less than –0.5, calculated as a subtraction of the mean zBICAMS at 7.5 years from the mean zBICAMS at 4.6 years.
Finally, the number of new relapses and disease duration (DD) were collected as additional clinical variables.
MR Imaging Data Acquisition and Analysis
A complete description of MR imaging data acquisition and analysis is available in a previous work.14 Briefly, MR imaging signs of Gd retention were qualitatively evaluated on unenhanced T1WIs, recording the presence of a visible bilateral hyperintensity affecting both DN (Fig 1). A quantitative MR imaging (qMRI) analysis of GBCA accumulation was achieved through the calculation of qMRI maps according to previous works21,22 and automatically extracting mean R1 values after the placement of 2 irregular bilateral ROIs on the axial section with the best representation of the DN (Fig 2).14
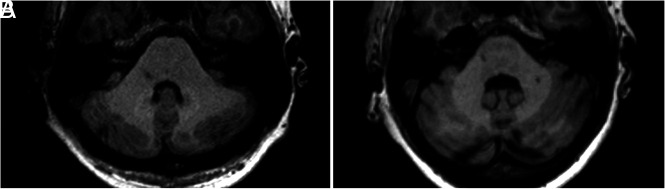
FIG 1.
Selected axial slices of unenhanced T1WI at the level of the DN. An example of increased (A) and absent (B) DN T1WI hyperintensity in 37- and 54-year-old female patients, both receiving a similar number of Gd administrations across time (n = 15 and n = 12, respectively) but a different molecule (gadopentetate dimeglumine and gadobutrol, respectively).
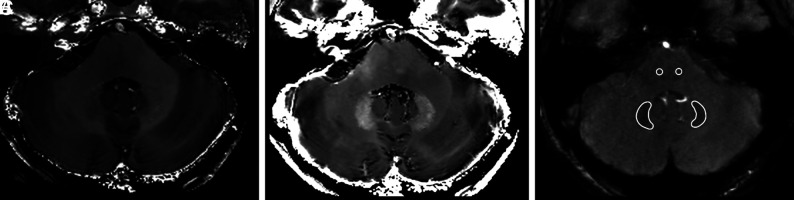
FIG 2.
Representative axial R1 (A) and R2* (B) maps at the level of the DN in a 28-year-old healthy male control. C, DN and brainstem ROI positioning on the flow-compensated gradient-echo sequence (TE2 = 22.14 ms, θ = 20°) used to obtain qMRI maps.
Finally, hyperintense lesions were detected and segmented on FLAIR images with a semiautomatic approach (Jim7; Xinapse Systems) to obtain lesion load volumes and for the inpainting procedure.23 By means of FSL SIENAX (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/SIENA), the T1WI volumes were processed to extract gray matter volume (GMV), normalized for the corresponding V-scaling factor, as a measure of cortical atrophy.
Statistical Analysis
To evaluate possible differences in terms of age and DD between stable and motor or cognitively worsened patients, we performed an independent 2-samples t test, while differences in terms of sex were tested through a χ2 test.
Possible differences in terms of ΔEDSS between patients with and without DN hyperintensity, along with possible differences of DN R1 values between stable and motor-worsened patients, were probed via the General Linear Model, accounting for potential confounding factors (age, sex, MS phenotype, DMT, GMV, lesion load, DD, and new relapses). Furthermore, the possible relationship between ΔEDSS and DN R1 values was tested via hierarchical multiple linear regression analysis, including clinical and demographic variables (sex, age, MS phenotype, DMT, DD, and new relapses) in the first block and MR imaging variables in the second one. For the cognitive evaluation, possible differences in terms of z scores of each BICAMS battery test between patients with and without DN hyperintensity, along with possible differences of DN R1 values between stable and cognitively worsened subjects, were tested using a GLM similar to the one previously described for the motor analyses. A similar hierarchical multiple linear regression analysis was also performed to test the possible relationship between BICAMS test z scores and clinical, demographic, and MR imaging variables.
Finally, the same analyses were also performed to compare ΔBICAMS between patients with and without DN hyperintensity and probe the possible relationship between DN R1 and the development of cognitive worsening or between ΔBICAMS and DN R1 values.
Methods and subsequent results of an additional subanalysis evaluating possible differences in terms of DN R1 values among subjects undergoing DMT changes with time are reported in the Online Supplemental Data.
All statistical analyses were performed by S.C. using the Statistical Package for the Social Sciences (Version 25.0; IBM), with a P = .05 set to indicate a statistically significant difference in the between-group comparison and regression analyses.
RESULTS
A complete list of demographic and clinical information of the studied population for motor and cognitive data is available in Tables 1 and 2, respectively.
Table 1:
Demographic and motor clinical variables of the subjects included in this studya
| Motor Examination | Baseline (n = 74) | Follow-Up (n = 63) |
|---|---|---|
| Age (mean) (range) (yr) | 36.1 (SD, 10.1) (21–62) | 44.4 (SD, 10.4) (28–69) |
| Sex (M/F) | 27:47 | 26:37 |
| DD (mean) (yr) | 9.8 (SD, 6.8) | 18.3 (SD, 7.0) |
| EDSS (median) (range) | 3.0 (1.5–6.5) | 2.5 (1.0–7.5) |
| Follow-up time from baseline (mean) (yr) | NA | 7.6 (SD, 0.6) |
| ΔEDSS (mean) | NA | –0.3 (SD, 0.9) |
| Clinical progression (progressed/stable) | NA | 6/57 |
Note:—NA indicates not applicable.
Motor worsening was defined if a subject showed a ΔEDSS ≥ 1 (for baseline EDSS ≤ 5.5) or ≥0.5 (for baseline EDSS > 5.5).
Table 2:
Demographic and cognitive clinical variables of the subjects included in this studya
| Cognitive Examination | First Follow-Up (n = 65) | Second Follow-Up (n = 32) |
|---|---|---|
| Age (mean) (range) (yr) | 36.5 (SD, 10.1) (21–62) | 45.9 (SD, 10.5) (29–61) |
| Sex (M/F) | 26:39 | 13:19 |
| DD (mean) (yr) | 15.0 (SD, 7.1) | 18.6 (SD, 7.7) |
| SDMT z score (mean) | –1.3 (SD, 1.2) | –1.1 (SD, 1.1) |
| BVMT z score (mean) | –0.9 (SD, 1.5) | –0.6 (SD, 1.4) |
| CVLT z score (mean) | –0.6 (SD, 1.4) | –0.4 (SD, 1.5) |
| BICAMS z score (mean) | –0.9 (SD, 1.1) | –0.7 (SD, 1.0) |
| FU time from baseline (mean) (yr) | 4.6 (SD, 1.0) | 7.5 (SD, 0.7) |
| ΔBICAMS (mean) | NA | –0.3 (SD, 0.8) |
| Cognitive impairment (impaired/preserved) | 40/25 | NA |
| Cognitive progression | NA | 5/32 (15.6%) |
Note:—FU indicates follow-up.
Cognitive impairment at the first time point was defined if a subject showed at least one of the z score values of equal or less than –1.5. Cognitive worsening at second time point was defined in case of ΔBICAMS equal or less than –0.5.
At baseline, 73/74 patients (98.7%) were in treatment with a DMT: fingolimod, n = 17; natalizumab, n = 28; interferon β-1a, n = 20; interferon β-1b, n = 7; and glatiramer acetate, n = 1). During a follow-up period of >7 years, 27.0% (17/63) of patients did not undergo therapeutic switches, whereas the remaining patients switched therapy once (55.5%, 35/63) or more (17.5%, 11/63) than once (natalizumab, n = 20; fingolimod, n = 14; cladribine, n = 8; ocrelizumab, n = 5; alemtuzumab, n = 4; interferon β-1a, n = 3; dimethyl fumarate, n = 3; interferon β-1b, n = 2; siponimod, n = 1; and peginterferon β-1a, n = 1).
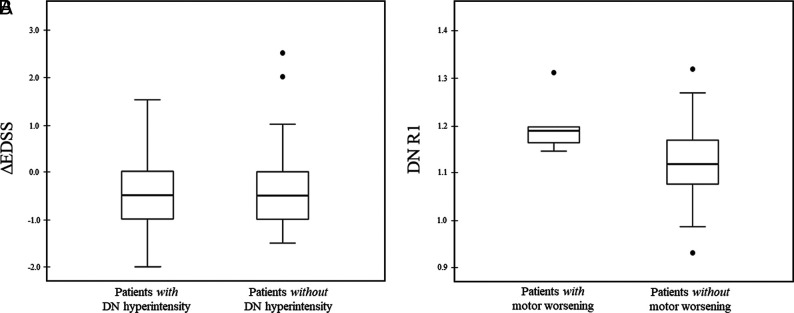
With reference to motor performances, 6 of 63 patients (9.5%) showed a motor worsening at follow-up, with 4 of these patients converting to a secondary-progressive course. Stable and worsened patients did not differ in terms of age (P = .39) or sex (P = .91), while a significant difference in DD (P = .04) emerged. When we compared subjects showing DN hyperintensity at baseline with patients without any detectable change on unenhanced T1WI, no significant difference emerged in terms of ΔEDSS (P = .14) (Fig 3A). Similarly, no significant difference was found in terms of DN R1 between stable subjects and patients showing motor worsening (P = .15) (Fig 3B).
FIG 3.
Boxplots showing ΔEDSS (A) and DN R1 values (B) of patients with and without a DN hyperintensity on unenhanced T1WI and motor worsening, respectively. Motor worsening was defined if a subject showed a ΔEDSS ≥ 1 (for baseline EDSS ≤ 5.5) or ≥0.5 (for baseline EDSS > 5.5). R1 values are expressed as s−1.
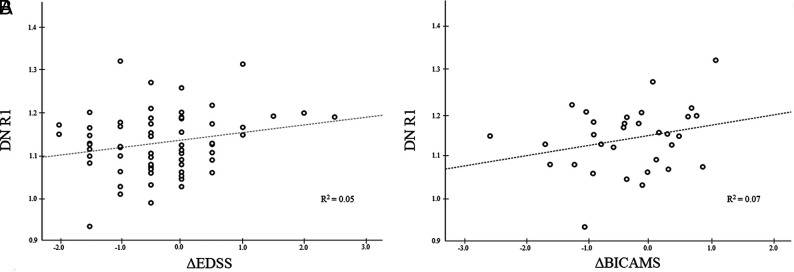
When we tested for a possible relation between DN R1 values and ΔEDSS, the regression model with clinical and demographic variables explained only 24.0% of the variance of ΔEDSS, whereas adding to the model lesion load and GMV increased the explained variance by 16.5% (40.5%, P = .005). When we evaluated independent predictors, the only significant effect was identified for GMV (P = .04), without any significant effect of DN R1 values in explaining the ΔEDSS variance (P = .21) (Fig 4A).
FIG 4.
Scatterplots showing the absence of a significant relation between DN R1 values and ΔEDSS (A) and ΔBICAMS (B), respectively. R1 values are expressed as s−1.
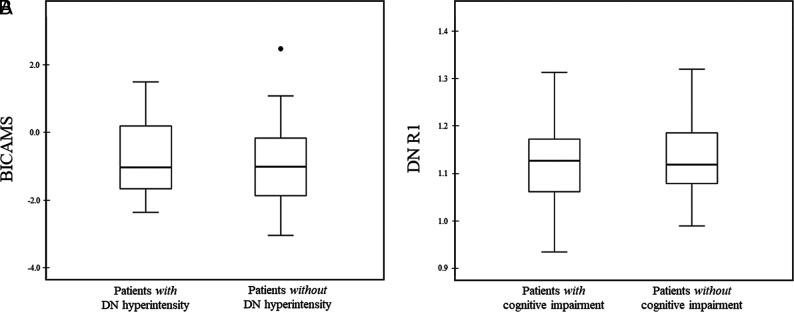
With reference to cognitive performances, 40 of 65 patients (61.5%) presented with cognitive impairment at a mean of 4.6 years of follow-up. These subjects did not differ from cognitively preserved patients in terms of age (P = .87), sex (P = .44), and DD (P = .37). Similarly, no significant differences were observed between patients with and without DN hyperintensity on MR imaging in terms of BICAMS z score (P = .92) or its individual components (P = .96 for the SDMT; P = .41 for the BVMT; P = .53 for the CVLT) (Fig 5A). Finally, the group of patients with cognitive impairment was not different in terms of mean DN R1 values (P = .26) compared with cognitively preserved subjects (Fig 5B).
FIG 5.
Boxplots showing BICAMS (A) and DN R1 values (B) of patients with and without DN hyperintensity on unenhanced T1WI and cognitive impairment, respectively. Cognitive impairment was defined if a subject showed at least 1 of the z score values equal or less than −1.5. R1 values are expressed as s−1.
When we investigated the relation between DN R1 values and BICAMS scores, the model explained 16.5% of the variance, without a significant effect of DN R1 in explaining BICAMS z scores (P = .30) or its components (P = .40 for the SDMT; P = .24 for the BVMT; P = .61 for the CVLT).
Finally, in the subset of patients with follow-up examinations available at 7.5 years, 5 of 32 patients (15.6%) showed cognitive worsening. These subjects were not different in terms of age (P = .96), sex (P = .60), DD (P = .19), or R1 values (P = .18) compared with stable patients. Similarly, there were no significant differences in terms of ΔBICAMS between patients with and without DN hyperintensity on MR imaging (P = .27) and no significant effect of DN R1 values in explaining the ΔBICAMS variance (P = .28) (Fig 4B).
DISCUSSION
A significant body of literature regarding Gd accumulation in tissues of patients with normal renal function has been published.2,3,6,9 Given that brain Gd retention occurs mainly in the DN,2,3,6,9 we investigated whether qualitative and quantitative MR imaging signs of Gd accumulation in this region would correlate with clinical changes with time.
With reference to motor performance, we found no significant difference in terms of DN R1 between stable and motor-worsened patients, in line with previous studies showing no significant association between GBCA exposure and the development of parkinsonism.10 Similarly, a case series in patients with glioblastoma multiforme24 who received at least 50 GBCA injections during 10 years did not identify any clinical impairment possibly related to Gd deposition. Additionally, our results expand findings from previous cross-sectional and short-term longitudinal studies in MS, reporting no association between motor disability and DN Gd deposition.11,25,26 Overall, these results are in line with the hypothesis of an absence of direct damage affecting the DN due to Gd deposition, given that this structure plays a key role in the physiology of the motor control loop27 and its involvement should, therefore, result in the development of harmful and disruptive motor symptoms similar to those observed in animal models in which direct damage to this structure has been induced.28
Exploring the cognitive counterpart of Gd retention, we did not find a significant association between cognitive impairment and mean DN R1 values, also in line with findings in most of the available literature.29,30
However, 2 studies in patients with MS12,25 previously reported a possible association between Gd retention and lower verbal fluency performance. One study12 observed an association between high signal DN intensity and low verbal fluency performances, and it might be tempting to quickly settle this matter by indicating, in the obvious advantages of qMRI, the most plausible explanation for these differences. Indeed, the same authors25 failed to confirm this association when evaluating quantitative R1 values. Nevertheless, in this same latter study, a mild correlation between poor information-processing speed (as measured by the SDMT) and DN R1 values25 was identified. Here, after analyzing a group of patients with MS with similar demographic and clinical features and a similar quantitative approach, we were not able to confirm this finding. These discrepancies across studies might be explained by several factors, and we fully agree that MS pathology might be confounding the results.25 Here, to address this issue and although we acknowledge that overcorrection might be a possible pitfall in statistical analysis,31 we have considered many known confounders that might account for changes in the SDMT results and failed to find any significant associations between SDMT and DN R1 values.
This result, corroborated by the absence of other significant associations within the cognitive domains assessed by the BICAMS, strengthens the hypothesis of an absence of a significant clinical impact of Gd retention in the brain, in line with recent preclinical observations showing no behavioral alterations in mice that developed T1WI hyperintensity on MR imaging after multiple injections of linear GBCA.32 Although the role of the cerebellum in language is well-recognized,33 verbal fluency tasks seem to be more related to the lateral portion of the hemispheres rather than the DN itself,33 as also confirmed by a coordinate-based activation likelihood estimation meta-analysis on brain activation during both phonemic and semantic verbal fluency tasks.34 Furthermore, given the above-mentioned role of the DN as a main relay of several different motor and cognitive loops, it seems very unlikely that among all the functions that might have been affected by Gd retention, only verbal fluency, which is characterized by a complex interplay of a variety of cognitive functions and brain areas,35 could have been involved. Similar considerations also apply to the correlation between increased DN T1WI hyperintensity and mild dysarthria observed in a different study,13 because dysarthria is usually related to hemispheric damage, with a preponderant right lateralization.36
Finally, we acknowledge that different from the motor analysis in which GMV proved to be an independent predictor of disability, we did not identify any MR imaging predictor of cognitive impairment. A possible explanation of this result should be researched in a more profound and prominent involvement of other brain areas, such as the deep gray matter,37-39 in explaining the development of cognitive deficits in MS.
This study has some limitations. As previously discussed, due to its retrospective nature, we were not able to investigate some features, such as dysarthria or verbal fluency, which could have been of interest; a direct investigation of the correlation between qMRI changes affecting the DN and verbal fluency or dysarthria is, therefore, warranted. Another limitation is the relatively low number of patients investigated, which might have limited the sensitivity toward smaller effect sizes as well as preventing us from performing a subgroup analysis comparing linear and macrocyclic GBCAs. Nevertheless, this represents a trade-off for the use of qMRI evaluation, that in change provides more accurate evaluation of Gd retention compared with qualitative conventional MR imaging.
CONCLUSIONS
Although characterized by these limitations, our results suggest that Gd accumulation, indirectly assessed via qualitative and qMRI parameters, is not associated with detectable clinical correlates in terms of global motor and cognitive worsening in MS.
ABBREVIATIONS:
- BICAMS
Brief International Cognitive Assessment for MS
- BVMT
Brief Visuospatial Memory Test
- CVLT
California Verbal Learning Test
- DD
disease duration
- DN
dentate nuclei
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- GBCA
gadolinium-based contrast agent
- Gd
gadolinium
- GLM
General Linear Model
- GMV
gray matter volume
- SDMT
Symbol Digit Modalities Test
- qMRI
quantitative MRI
Footnotes
A. Scaravilli and M. Tranfa contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Gulani V, Calamante F, Shellock FG, et al. ; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017;16:564–70 10.1016/S1474-4422(17)30158-8 [DOI] [PubMed] [Google Scholar]
- 2.Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–41 10.1148/radiol.13131669 [DOI] [PubMed] [Google Scholar]
- 3.Quattrocchi CC, Mallio CA, Errante Y, et al. Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol 2015;50:470–72 10.1097/RLI.0000000000000154 [DOI] [PubMed] [Google Scholar]
- 4.Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol 2021;20:653–70 10.1016/S1474-4422(21)00095-8 [DOI] [PubMed] [Google Scholar]
- 5.Brisset JC, Kremer S, Hannoun S, et al. New OFSEP recommendations for MRI assessment of multiple sclerosis patients: special consideration for gadolinium deposition and frequent acquisitions. J Neuroradiol 2020;47:250–58 10.1016/j.neurad.2020.01.083 [DOI] [PubMed] [Google Scholar]
- 6.Errante Y, Cirimele V, Mallio CA, et al. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 2014;49:685–90 10.1097/RLI.0000000000000072 [DOI] [PubMed] [Google Scholar]
- 7.Robert P, Lehericy S, Grand S, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol 2015;50:473–80 10.1097/RLI.0000000000000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA Drug Safety Communication: FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging (MRI). May 18, 2017. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-evaluating-risk-brain-deposits-repeated-use-gadolinium-based. Accessed December 1, 2022
- 9.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;275:772–82 10.1148/radiol.15150025 [DOI] [PubMed] [Google Scholar]
- 10.Welk B, McArthur E, Morrow SA, et al. Association between gadolinium contrast exposure and the risk of parkinsonism. JAMA 2016;316:96–98 10.1001/jama.2016.8096 [DOI] [PubMed] [Google Scholar]
- 11.Cocozza S, Pontillo G, Lanzillo R, et al. MRI features suggestive of gadolinium retention do not correlate with Expanded Disability Status Scale worsening in multiple sclerosis. Neuroradiology 2019;61:155–62 10.1007/s00234-018-02150-4 [DOI] [PubMed] [Google Scholar]
- 12.Forslin Y, Shams S, Hashim F, et al. Retention of gadolinium-based contrast agents in multiple sclerosis: retrospective analysis of an 18-year longitudinal study. AJNR Am J Neuroradiol 2017;38:1311–16 10.3174/ajnr.A5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kühn I, Maschke H, Großmann A, et al. Dentate-nucleus gadolinium deposition on magnetic resonance imaging: ultrasonographic and clinical correlates in multiple sclerosis patients. Neurol Sci 2022;43:2631–39 10.1007/s10072-021-05702-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tedeschi E, Palma G, Canna A, et al. In vivo dentate nucleus MRI relaxometry correlates with previous administration of gadolinium-based contrast agents. Eur Radiol 2016;26:4577–84 10.1007/s00330-016-4245-2 [DOI] [PubMed] [Google Scholar]
- 15.Wattjes M, Rovira À, Miller D, et al.. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015;11:597–606 10.1038/nrneurol.2015.157 [DOI] [PubMed] [Google Scholar]
- 16.Wattjes MP, Ciccarelli O, Reich DS, et al. ; Magnetic Resonance Imaging in Multiple Sclerosis study group; Consortium of Multiple Sclerosis Centres; North American Imaging in Multiple Sclerosis Cooperative MRI Guidelines Working Group. MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol 2021;20:653–70 10.1016/S1474-4422(21)00095-8 [DOI] [PubMed] [Google Scholar]
- 17.Benedict RH, Amato MP, Boringa J, et al. Brief International Cognitive Assessment for MS (BICAMS): international standards for validation. BMC Neurol 2012;12:55 10.1186/1471-2377-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goretti B, Niccolai C, Hakiki B, et al. The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): normative values with gender, age and education corrections in the Italian population. BMC Neurol 2014;14:171 10.1186/s12883-014-0171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocozza S, Pontillo G, Russo C, et al. Cerebellum and cognition in progressive MS patients: functional changes beyond atrophy? J Neurol 2018;265:2260–66 10.1007/s00415-018-8985-6 [DOI] [PubMed] [Google Scholar]
- 20.Meijer KA, van Geest Q, Eijlers AJC, et al. Is impaired information processing speed a matter of structural or functional damage in MS? Neuroimage Clin 2018;20:844–50 10.1016/j.nicl.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrelli P, Palma G, Tedeschi E, et al. Improving signal-to-noise ratio in susceptibility weighted imaging: a novel multicomponent non-local approach. PloS One 2015;10:e0126835 10.1371/journal.pone.0126835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palma G, Tedeschi E, Borrelli P, et al. A novel multiparametric approach to 3D quantitative MRI of the brain. PloS One 2015;10:e0134963 10.1371/journal.pone.0134963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp 2012;33:2062–71 10.1002/hbm.21344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vymazal J, Krámská L, Brožová H, et al. Does serial administration of gadolinium-based contrast agents affect patient neurological and neuropsychological status? Fourteen-year follow-up of patients receiving more than fifty contrast administrations. J Magn Reson Imaging 2020;51:1912–13 10.1002/jmri.26948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forslin Y, Martola J, Bergendal Å, et al. Gadolinium retention in the brain: an MRI relaxometry study of linear and macrocyclic gadolinium-based contrast agents in multiple sclerosis. AJNR Am J Neuroradiol 2019;40:1265–73 10.3174/ajnr.A6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zivadinov R, Bergsland N, Hagemeier J, et al. Cumulative gadodiamide administration leads to brain gadolinium deposition in early MS. Neurology 2019;93:e611–23 10.1212/WNL.0000000000007892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 2000;31:236–50 10.1016/S0165-0173(99)00040-5 [DOI] [PubMed] [Google Scholar]
- 28.Vilis T, Hore J. Effects of changes in mechanical state of limb on cerebellar intention tremor. J Neurophysiol 1977;40:1214–24 10.1152/jn.1977.40.5.1214 [DOI] [PubMed] [Google Scholar]
- 29.Mallio CA, Quattrocchi CC, Rovira À, et al. Gadolinium deposition safety: seeking the patient's perspective. AJNR Am J Nuroradiol 2020;41:944–46 10.3174/ajnr.A6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallio CA, Piervincenzi C, Gianolio E, et al. Absence of dentate nucleus resting-state functional connectivity changes in nonneurological patients with gadolinium-related hyperintensity on T(1)-weighted images. J Magn Reson Imaging 2019;50:445–55 10.1002/jmri.26669 [DOI] [PubMed] [Google Scholar]
- 31.Lee PH. Should we adjust for a confounder if empirical and theoretical criteria yield contradictory results? A simulation study. Sci Rep 2014;4:6085 10.1038/srep06085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akai H, Miyagawa K, Takahashi K, et al. Effects of gadolinium deposition in the brain on motor or behavioral function: a mouse model. Radiology 2021;301:409–16 10.1148/radiol.2021210892 [DOI] [PubMed] [Google Scholar]
- 33.Murdoch BE. The cerebellum and language: historical perspective and review. Cortex 2010;46:858–68 10.1016/j.cortex.2009.07.018 [DOI] [PubMed] [Google Scholar]
- 34.Wagner S, Sebastian A, Lieb K, et al. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci 2014;15:19 10.1186/1471-2202-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson J. Verbal fluency. In: Kreutzer JS, DeLuca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology. Springer-Verlag; 2011:2603–06 [Google Scholar]
- 36.Ackermann H, Hertrich I. The contribution of the cerebellum to speech processing. J Neurolinguistics 2000;13:95–116 10.1016/S0911-6044(00)00006-3 [DOI] [Google Scholar]
- 37.Pontillo G, Penna S, Cocozza S, et al. Stratification of multiple sclerosis patients using unsupervised machine learning: a single-visit MRI-driven approach. Eur Radiol 2022;32:5382–91 10.1007/s00330-022-08610-z35284989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007;69:1213–23 10.1212/01.wnl.0000276992.17011.b5 [DOI] [PubMed] [Google Scholar]
- 39.Petracca M, Pontillo G, Moccia M, et al. Neuroimaging correlates of cognitive dysfunction in adults with multiple sclerosis. Brain Sci 2021;11:346 10.3390/brainsci11030346 [DOI] [PMC free article] [PubMed] [Google Scholar]